MIR - Current research and publications
Find out below what the research within the division of Medical Inflammation Research (MIR) is aimed at. Also listed are examples of research projects and an overview of our collaborations and recent publications.
The aims of our research are:
- To identify and functionally analyse the genes that control animal models for chronic inflammatory diseases, mainly using models for rheumatoid arthritis.
- To use the animal models not only for understanding the basic mechanism of autoimmune disease and for developing new diagnostic, preventive and therapeutic strategies.
- To understand the role of MHC class II genes in explaining the immune specificity of autoimmune disease.
We have different research projects within our division. Below, some examples of such projects.
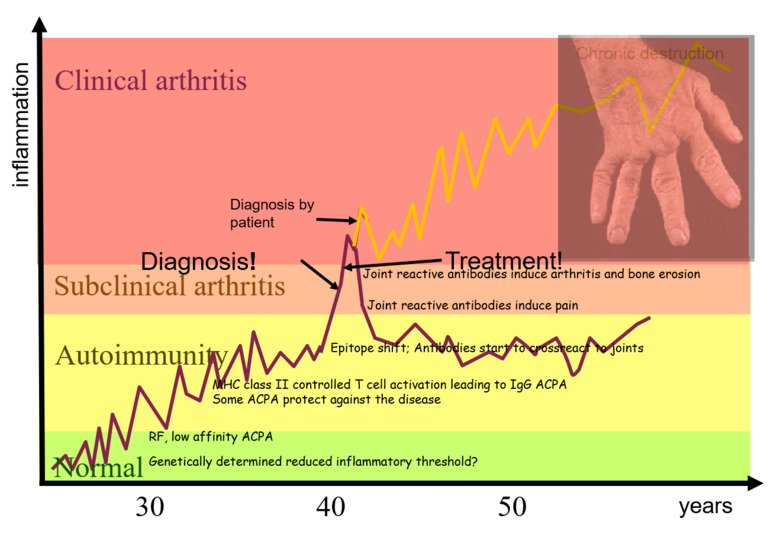
Diagnostics project: JointID clinical treatment research – a new unique diagnostic test for Rheumatoid Arthritis
Inflammation in cartilaginous joints occurs in many common diseases such as rheumatoid arthritis (RA), osteoarthritis (OA) and psoriasis arthritis (PsA). It is known that antibodies to modified proteins (anticitrullinated protein antibodies and rheumatoid factors) are useful for predicting and classifying RA. It has however been difficult to identify specific and useful biomarkers derived from the joint inflammation itself.
We have focused on the immune response to joint cartilage proteins, in particular type II collagen (CII). This is directed to exposed triple helical structures, as well as to structures modified e.g. by citrullination. The response is connected with the onset of the RA and provides very sensitive biomarkers for joint inflammation.
We have three main goals:
- To develop a multiplex antibody diagnostic kit with post-translationally modified (citrullinated, carbamoylated, nitrosylated, oxidized and glycosylated) and conformational protein epitopes. These epitopes are selected to be of importance for development of arthritis in animal models. The purpose is to allow diagnosis of RA before disease onset and to help in determination of the type of therapy. The assay will also be used to select RA patients suitable for vaccination.
- To develop a diagnostic kit to identify T cells specific for glycosylated CII. It will be used to predict and monitor vaccination effects of pre-RA and RA patients.
- To extend the antibody kit with additional joint-related epitopes useful for diagnosing additional joint inflammatory diseases, including common diseases like OA and PsA.
Coordinator

Main PI
Prof. Rikard Holmdahl.
Cooperation
Prof. Inger Gjertsson, Göteborg University; Prof. Jan Kihlberg, Uppsala University
Supported by
- Swedish Foundation for Strategic Research (SSF) 2015 – 2020 (Dnr: RB13-0156, 35,000,000 SEK)
Vaccine project: Developing vaccines to cure autoimmune diseases
The next step in the treatment of autoimmune disease will be to prevent and cure, rather than only treating the symptoms. We focus on vaccine development to both prevent and treat rheumatoid arthritis (RA) but subsequently also other autoimmune diseases.
Vaccines have not yet been developed to any autoimmune disease, but we have several unique findings that will be tested clinically in RA. Our patent protected approach is to induce regulatory T cells by administration of a protein complex with the product of a strongly associated RA gene together with a protein fragment from collagen type II in joint cartilage that is a recognized by T cells in RA.
We have established new unique mouse strains with the human genes of interest, allowing development and validation of new vaccines not only for RA but for other autoimmune diseases as well. We have also developed new technology that allows modification of peptide structure; glycosylation and protein carrier, to develop improved personalized variants vaccines.
Coordinator
Main PI
Prof. Rikard Holmdahl
Cooperation
Prof. Roman Zubarev, Division of Chemistry I and chemical proteomics platform, Karolinska Institutet; Prof. Jan Kihlberg, Uppsala University; Dr. Kajsa Wing, Division of Medical inflammation Research, Karolinska Institutet
Supported by

- Swedish Science Council (VR), 2018-2022, (Dnr. 2017-06104, 19,000,000 SEK)
- Familjen Erling-Perssons Stiftelse, 2018-2020 (2017-10-09, 9,000,000 SEK)
Ncf1 project: Changing the view on autoimmune disease based on positional cloning of the Ncf1 gene
We have identified several major causative polymorphisms in mouse and rat models of autoimmune disease and are now investigating their functional role. One of these projects concerns Ncf1, a component of the NOX2 complex, which induces reactive oxygen species (ROS).
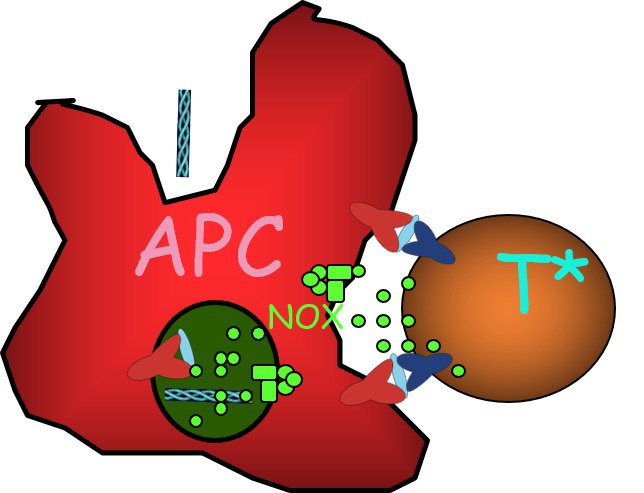
We found that Ncf1 alleles causing low production of reactive oxygen species (ROS) is a major cause of autoimmune disease as well as being a pronounced risk behavior, explaining the natural selection. In addition ROS is likely to regulate the immune response to cancer cells.
The Ncf1 locus is highly polymorphic and has in fact not been sequenced in humans, due to copy number variation, and is therefore not included in genome wide association studies. However, we have found that copy number variation and amino acid polymorphism is strongly associated with both rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE).
Based on our original finding and many years of combined efforts in both experimental animals and humans we have now established a unique platform and strategy to reveal a critical part of the pathogenesis of autoimmune disease. We have established CRISPR mutated and conditionally controlled Ncf1 mouse strains.
With models for RA and SLE we have also confirmed that the pathways are conserved between mice and humans. We have preliminary evidence of direct links to major pathways, such as interferon signaling and T and B cell receptor signaling. We intent to identify the immune regulatory cells expressing Ncf1 that play a critical role in the downstream pathway of autoimmune disease such as RA and SLE, but also in cancer. Also to determine the signaling oxidative and antioxidant pathways in the regulatory Ncf1 expressing cells as well as in cells exposed to secreted ROS.
This project will provide new insights into key pathogenic mechanisms based on redox regulation, leading to autoimmune and malignant diseases.
Coordinator
Main PI
Prof. Rikard Holmdahl
Cooperation
Prof. Elias Arnér, Division of Biochemistry, Karolinska Institute; Prof. Roman Zubarev, Division of Chemistry I and chemical proteomics platform, Karolinska Institute
Supported by

- Knut och Alice Wallenbergs Stiftelse, 2020 – 2024 (Dnr KAW 2019.0059, 36,500,000 SEK)
Details about the project funding: "Genfynd kan bana väg för vaccin mot autoimmuna sjukdomar" - Swedish Science Research Council (VR), 2020-2024 (Dnr. 2019-01209, 9,000,000 SEK)
EU MSCA project COSMIC: Combatting disorders of adaptive Immunity with systems medicine (COSMIC)

Grant no: 765158
Period: 2018 – 2021
Budget: 486,710 euro
Marie Curie fellows
Alexander Krämer
MC FellowProject title: Identification of VDJ repertoire related to arthritis in humans.

Ana Coelho
MC FellowProject title: Identification of VDJ repertoire related to arthritis in mouse.
Psoriasis vulgaris project: New therapy and diagnostics of psoriasis vulgaris and psoriatic arthritis based on new animal models
Psoriasis vulgaris (PsV) is an immune mediated disease of the skin, which is associated in one third of the patients with the development of psoriatic arthritis (PsA). PsV is a complex chronic disease driven by adaptive and innate lymphocytes involving the IL-17/22/23 signaling pathway. It is associated with the PSORS1 locus within the major histocompatibility complex (MHC) region, containing non-classical MHC genes regulating IL-17 producing innate lymphocytes. Besides the strong influence of the MHC region, a locus with the inducible nitric oxide synthase (NOS2) gene is associated with PsV/PsA susceptibility and the NOS2 locus regulate the exposure of various reactive oxygen species (ROS). Thus, genetic evidence argues for that non-classical MHC genes involved in the innate immune responses are regulated by RNS/ROS mediated functions.
The environmental causes of the PsV/ PsA in humans is not known but some sort of environmental challenges triggering the immune system in pathogenic way is likely. And it is likely that an immune adjuvant type of exposure, mediated by infections, pollution or smoking, will trigger or promote disease. Our project is based on the unique discovery that mannan exposure could induce the development of a disease in mice mimicking PsV and PsA. In this project, we will use the MIP model to investigate PsV and PsA with the aim to develop new type of diagnostics and treatments.
The mechanisms by which mannan triggers disease is not known but is likely involving activation of inflammatory cells through CLRs. These receptors could be both inflammatory and anti-inflammatory, partially dependent on their ability to activate the NOX2 complex that could induce reactive oxygen species (ROS), protecting disease development in the MIP model. Based on this, we will use mouse strains with and without the Ncf1 mutation to determine whether the observed therapeutic effect is dependent of the proposed mechanisms of action through the NOX2 complex. We will also use the model to facilitate the development of better diagnostics for PsV and PsA, with developed a multiplex peptide-based antibody test to investigate the ongoing autoantibody responses in in autoimmune diseases.
Coordinator
Main PI
Prof. Rikard Holmdahl
Supported by
- Leo Foundation 2023- 2025 (LF-OC-22-001023, DKK 3,622,500)
- Psoriasisförbundet 2021-2022 (2021-2022, 200,000 SEK)
Collaborations
- Partner in network for Cancer Redox - targeting redox pathways for improved cancer therapy, Coord. Prof. Elias Arnér, Biochemistry, Karolinska Institute, Supported by Knut och Alice Wallenbergs Stiftelse, 2016 – 2021 (Dnr. 2015.0063)
- Partner in network for A new unique diagnostics test for Rheumatoid Arthritis, Coord Prof. Inger Gjertsson, Göteborg university, supported by Swedish Research council, 2017 – 2019 (Dnr 2016-00288)
- Longstanding collaboration with Xian Jiaotong university and the 2nd affiliated hospital in Xian, China.
- Cooperation with Fraunhofer research institute in Frankfurt Am Main.
- Guangdong province team project with Southern Medical university in Guangzhou, China
- Cooperation project with Prof. Gregg Fields, Florida Atlantic University, funded by NIH 2021-2023 (KR-K210)
- Cooperation with BioInvent international AB, Lund
- Cooperation with Sichuan University, West China Hospital in Chendu, China
Selected recent publications
- Aoun M, Coelho A, Krämer A, Saxena A, Sabatier P, Beusch C, Lönnblom E, Geng M, Do N, Xu Z, Zhang J, He Y, Castillo L, Abolhassani H, Xu B, Viljanen J, Rorbach J, Lahore G, Gjertsson I, Kastbom A, Sjowall C, Kihlberg J, Zubarev R, Burkhardt H, Holmdahl R*. Antigen presenting autoreactive B cells activate regulatory T cells and suppress autoimmune arthritis in mice. J Exp Med. 2023, 220 (11)
- Xu Z, Xu B, Lundström S, Giró A, Zhao D, Martin M, Lönnblom E, Li Q, Krämer A, Ge C, Cheng L, Liang B, Tong D, Stawikowska R, Blom A, Fields G, Zubarev R, Holmdahl R*. A subset of type-II collagen-binding antibodies prevents experimental arthritis by inhibiting FCGR3 signaling in neutrophils. Nat Commun. 2023,14, 5949
- Urbonaviciute V, Romero-Castillo L, Xu B, Luo H, Schneider N, Weisse S, Do NN, Oliveira-Coelho A, Fernandez Lahore G, Li T, Sabatier P, Beusch CM, Viljanen J, Zubarev RA, Kihlberg J, Bäcklund J, Burkhardt H, Holmdahl R*. Therapy targeting antigen-specific T cells by a peptide-based tolerizing vaccine against autoimmune arthritis. Proc Natl Acad Sci U S A. 2023 Jun 20;120(25)
- Lönnblom E, Leu Agelii M, Sareila O, Cheng L, Xu B, Viljanen J, Hafström I, Andersson ML, Bergström G, Hultgård Ekwall AK, Rudin A, Kastbom A, Sjöwall C, Jacobsson LT, Kihlberg J, Gjertsson I, Holmdahl R*. Autoantibodies to disease-related proteins in joints as novel biomarkers for the diagnosis of rheumatoid arthritis. Arthritis Rheumatol. 2023 Jul;75(7):1110-1119
- He Y, Ge C, Moreno-Giró À, Xu B, Beusch CM, Sandor K, Su J, Cheng L, Lönnblom E, Lundqvist C, Slot LM, Tong D, Urbonaviciute V, Liang B, Li T, Lahore GF, Aoun M, Malmström V, Rispens T, Ernfors P, Svensson CI, Scherer HU, Toes REM, Gjertsson I, Ekwall O, Zubarev RA, Holmdahl R*. A subset of antibodies targeting citrullinated proteins confers protection from rheumatoid arthritis. Nat Commun. 2023 14:691
- Li Y, Li Z, Nandakumar KS, Holmdahl R*. Human NCF190H Variant Promotes IL-23/IL-17-Dependent Mannan-Induced Psoriasis and Psoriatic Arthritis. Antioxidants (Basel). 2023 Jun 27;12(7):1348
- Luo H, Urbonaviciute V, Saei AA, Lyu H, Gaetani M, Végvári Á, Li Y, Zubarev RA, Holmdahl R*. NCF1-dependent production of ROS protects against lupus by regulating plasmacytoid dendritic cell development and functions. JCI Insight. 2023 Apr 10; 8(7): e164875.
- Li T, Ge C, Krämer A, Sareila O, Leu Agelii M, Johansson L, Forslind K, Lönnblom E, Yang M, Xu B, Li Q, Cheng L, Bergström G, Fernandez G, Kastbom A, Rantapää-Dahlqvist S, Gjertsson I, Holmdahl R*. Pathogenic antibody response to glucose-6-phosphate isomerase targets a modified epitope uniquely exposed on joint cartilage. Ann Rheum Dis. 2023 Jun;82(6):799-808
- Ge C, Cienciala S, Xu B, Dobritzsch D, Viljanen J, Kihlberg J, Do NN, Schneider N, Lanig H, Holmdahl R*, Burkhardt H*: Key interactions in the trimolecular complex consisting of the rheumatoid arthritis-associated DRB1*04:01 molecule, the major glycosylated collagen II peptide, and the T-cell receptor. Ann Rheum Dis 2022 Apr; 81(4): 480–489
- Kissel T, Ge C, Hafkenscheid L, Slot LM, Cavallari M, Kwekkeboom JC, He Y, van Schie KA, Vergroesen RD, Kampstra ASB, Reijm S, Stoeken-Rijsbergen G, Heitman LH, Xu B, Pruijn GJM, Wuhrer M, Rispens T, Huizinga TWJ, Scherer HU, Reth M, Holmdahl R*, Toes REM* : N-linked glycosylation of the immunoglobulin variable 1 domain affects antigen binding and autoreactive B-cell activation. Sci Adv, 09 Feb 2022, 8(6)
- Fernandez LahoreG, Förster M, Johannesson, M, SabatierP, Lönnblom E, AounM, He Y, NandakumarKS, ZubarevRA, HolmdahlR*: Polymorphism in estrogen receptor binding site causes CD2-dependent sex bias in T cell autoimmune diseases. Nat Commun 2021 Sep 22;12(1):5565
- Norin U*, Rintisch C, Meng L, Forster F, Ekman D, Tuncel J, Klocke K, Bäcklund J, Yang M, Bonner MY, Lahore GF, James J, Shchetynsky K, Bergquist M, Gjertsson I, Hubner N, Bäckdahl L, Holmdahl R*. Endophilin A2 deficiency protects rodents from autoimmune arthritis by modulating T cell activation. Nat Commun. 2021 Jan 27;12(1):610
- Fernandez Lahore G, Raposo B, Lagerquist M, Ohlsson C, Sabatier P, Xu B, Aoun M, James J, Cai X, Zubarev RA, Nandakumar KS, Holmdahl R*. Vitamin D3 receptor polymorphisms regulate T cells and T cell-dependent inflammatory diseases. Proc Natl Acad Sci U S A. 2020 Oct 6;117(40):24986-24997
- Zhu W, Lönnblom E, Förster M, Johannesson M, Tao P, Meng L, Lu S, Holmdahl R*. Natural polymorphism of Ym1 regulates pneumonitis through alternative activation of macrophages. Sci Adv. 2020 Oct 21;6(43)
- Bersellini Farinotti A, Wigerblad G, Nascimento D, Bas DB, Morado Urbina C, Nandakumar KS, Sandor K, Xu B, Abdelmoaty S, Hunt MA, Ängeby Möller K, Baharpoor A, Sinclair J, Jardemark K, Lanner JT, Khmaladze I, Borm LE, Zhang L, Wermeling F, Cragg MS, Lengqvist J, Chabot-Doré AJ, Diatchenko L, Belfer I, Collin M, Kultima K, Heyman B, Jimenez-Andrade JM, Codeluppi S, Holmdahl R*, Svensson CI*. Cartilage-binding antibodies induce pain through immune complex-mediated activation of neurons. J Exp Med. 2019 Aug 5;216(8):1904-1924
- Raposo B, Merky P, Lundqvist C, Yamada H, Urbonaviciute V, Niaudet C, Viljanen J, Kihlberg J, Kyewski B, Ekwall O, Holmdahl R*, Bäcklund J*. T cells specific for post-translational modifications escape intrathymic tolerance induction. Nat Commun. 2018 Jan 24;9(1):353
- Olsson LM, Johansson ÅC, Gullstrand B, Jönsen A, Saevarsdottir S, Rönnblom L, Leonard D, Wetterö J, Sjöwall C, Svenungsson E, Gunnarsson I, Bengtsson AA, Holmdahl R*. A single nucleotide polymorphism in the NCF1 gene leading to reduced oxidative burst is associated with systemic lupus erythematosus. Ann Rheum Dis. 2017 Sep;76(9):1607-1613
- Olofsson P, Holmberg J, Tordsson J, Lu S, Akerström B, Holmdahl R*. Positional identification of Ncf1 as a gene that regulates arthritis severity in rats. Nat Genet. 2003 Jan;33(1):25-32
- Vingsbo-Lundberg C, Nordquist N, Olofsson P, Sundvall M, Saxne T, Pettersson U, Holmdahl R*. Genetic control of arthritis onset, severity and chronicity in a model for rheumatoid arthritis in rats. Nat Genet. 1998 Dec;20(4):401-4
- Sundvall M, Jirholt J, Yang HT, Jansson L, Engström A, Pettersson U, Holmdahl R*. Identification of murine loci associated with susceptibility to chronic experimental autoimmune encephalomyelitis. Nat Genet. 1995 Jul;10(3):313-7
