Circadian rhythms in the cochlea
Auditory function has been shown to be influenced by the circadian system. Circadian rhythms are driven by the circadian clock machinery (biological clocks) to ensure that the organism anticipates and adapts to temporal changes in the environment.
The light/dark cycle has a strong influence on biological clocks, which is most obvious in the form of sleep-wake cycle. However, many other biological functions such as behavior, metabolic function, cardiovascular, endocrine, digestive and immune systems are under the control of the circadian system.
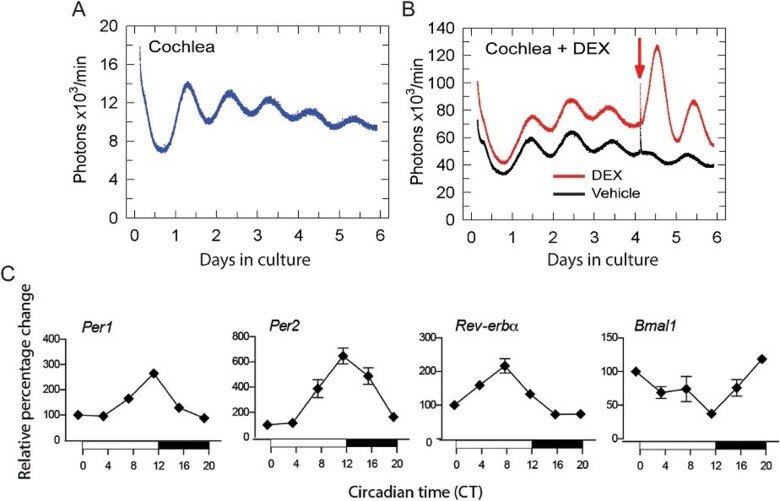
We discovered that the peripheral auditory system, the cochlea, is regulated by a molecular circadian clock, which opened an exceptional opportunity for understanding unique features of the auditory system that were previously unknown. A robust molecular circadian clock machinery including the core clock genes Per1, Per2, Bmal1, and Rev-Erb-alpha, was identified in the cochlea.
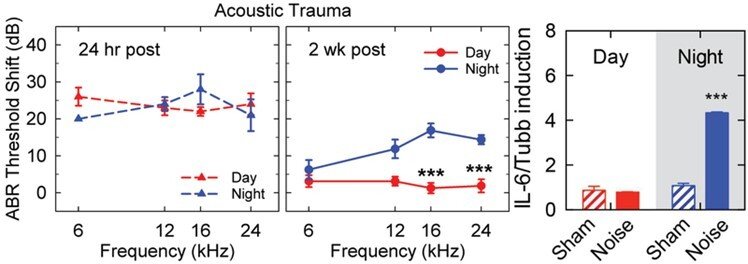
We have found that the same noise exposure applied during daytime (inactive phase) or nighttime (active phase) in mice causes greater pathophysiological and morphological consequences after night noise trauma. This unique noise paradigm causes similar hearing threshold shifts 24h post-noise exposure but while the day noise-exposed mice fully recover their hearing thresholds, the night noise-exposed animals show permanent damage and thus impaired recovery. Interestingly, in absence of the SCN or the adrenal glands, night noise sensitivity is no longer evident.
Using RNAseq we recently identified 7211 genes (49%) in the cochlea that have circadian expression and a large proportion of these regulate cell signaling, hormone secretion, and inflammation4. Nearly ⅔ of these genes show peak expression at nighttime, a finding that can only be determined when performing kinetic analyses.
The presence of immune cells and the involvement of immune responses (cytokines, activated immune cells and recruitment to the cochlea) after noise damage has been previously reported. A large body of evidence shows the activation of macrophages in the cochlea after noise trauma and that macrophage recruitment into the cochlea may enhance damage. However, there are critical gaps in knowledge of how circadian rhythms affect immune function in the cochlea. Our preliminary findings point towards a greater inflammation occurring in mice after noise trauma during their active phase (nighttime), suggesting that the existing published literature has underestimated the impact of noise on inflammatory processes due to investigating mice during their inactive phase (daytime). It is thus important to know how inflammation influences (positively or negatively) auditory recovery after noise trauma.
