Electron Microscopy Core Facility (EMil) - our offer
We have extensive experience in conventional sample preparation for both TEM and SEM, preparation for immunohistochemistry, morphometric analysis, negative staining, low temperature techniques such as low temperature embedding and cryo-sectioning. The unit manages two transmission electron microscopes TEM (Hitachi HT7700 and Hitachi HT7800) and one scanning electron microscope (Zeiss Ultra 55).
Our services
Ultrathin sectioning – TEM
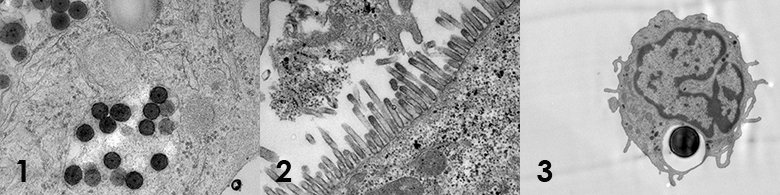
Ultrathin sectioning TEM, a preparation method for ultrastructural analysis of larger biological specimens such as various tissues, cells and bacteria. It involves chemical fixation, dehydration, plastic embedding and ultrathin sectioning. Typical questions are for example general morphology, cellular uptake of nanoparticles, morphological changes of intracellular organelles due to treatment by e.g. a chemical, siRNA etc. The method is routinely used in ultrastructural pathology for the diagnosis of renal, muscle and cilia related diseases.
Negative stain TEM
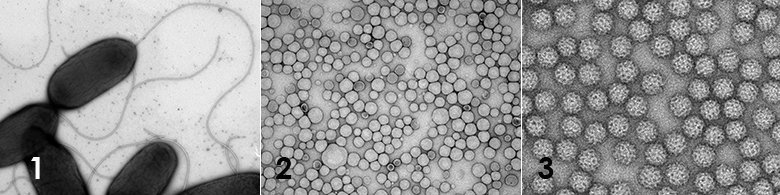
Negative stain TEM, a quick-and-dirty method to assess the morphology, purity, size, aggregation state etc. of suspensions containing various nanoparticles such as protein complexes, virus particles, inorganic nanoparticles etc. The method can also be used to analyse suspensions of larger biological specimens such as bacteria and mycoplasma to assess the size and shape but also presence of extracellular organelles such as cilia, fimbriae and flagellum. Membranous materials such as liposomes, LNP’s, exosomes and virosomes can also be analysed using negative stain TEM.
Scanning electron microscopy (SEM)
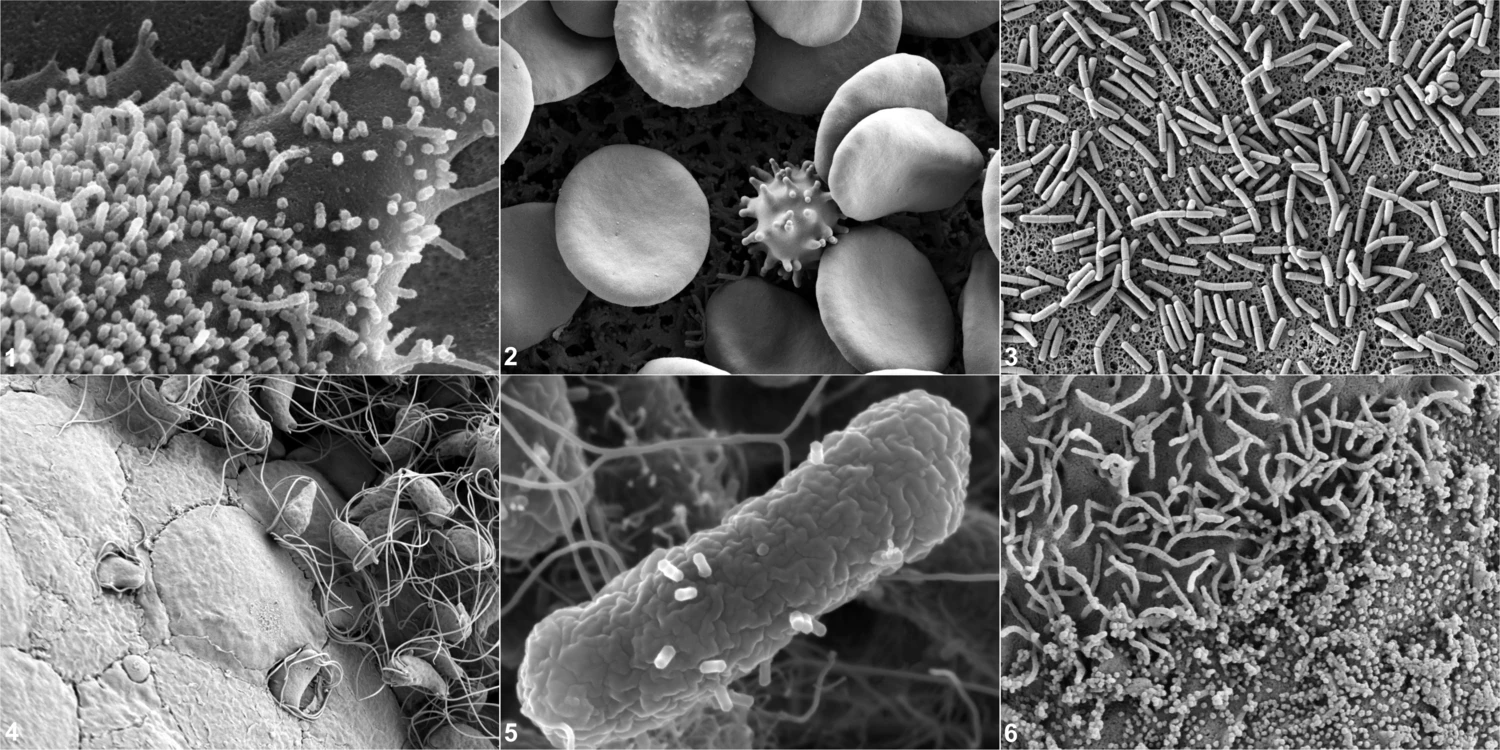
Scanning electron microscopy of biological specimens typically involves a topographical representation of the specimen that in many scientific questions can give unique and very informative morphological information regarding the overall specimen morphology. Similar to TEM, sample preparation involves chemical fixation and dehydration and prior to imaging the specimen is sputtered using e.g. platinum. The scattered electrons originating from the metal coating will then be detected when irradiated by a scanning beam of incident electrons.
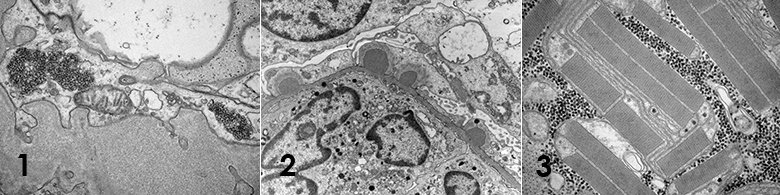
Clinical electron microscopy
The EM facility also performs clinical services of primarily renal biopsies for the diagnostics of kidney related diseases but other diagnostics are performed such as cilia related diseases.
Equipment
TEM
TEM images are acquired using a Hitachi HT7800 or a HT7700 120kV transmission electron microscope where the HT7800 is equipped with 20MPx Xarosa CMOS and the HT7700 a 4MPx Veleta CCD camera.
SEM
SEM images are acquired using a Zeiss Gemini Ultra 55 field emission equipped with on-axis SE detector (InLens SE), Everhart-Thornley SE detector (SE2), energy selective backscatter (ESB), angle selective backscatter (ASB) or STEM detector.
Sample preparation
For the TEM and SEM sample preparation we have access to Leica EM AFS2 FSP, EM Tissue Processor and two UC7 ultramicrotomes and cryo-ultramicrotome for the thin sectioning of tissue and cell specimens. SEM sample preparation is done using a Leica CPD300 critical point dryer and a Qourum Q150T ES sputter coater/carbon evaporator. For grid hydrophilization we have access to a Pelco easiGlow™.
External use of the core facility
EMil can offer services without a commissioned/contract research or collaborative research agreement, in accordance with the Swedish governmental ordinance (2022:1378) on fees for research infrastructure.
Find out more about external use of KI's core facilities
