OPENCORONA
The OPENCORONA consortia is developing a vaccine that protects against SARS-CoV-2 infection and/or disease in a phase I clinical trial.
Several human coronavirus immunotherapy or vaccines have been developed, mainly based on the Spike protein. Genetic analysis shows that the SARS-CoV-2 envelope and receptor binding domain only has a 75% homology with other human coronaviruses, and mutations accumulate in this region. We therefore look at including additional viral proteins that are more genetically conserved. We use the DNA vaccine platform which is a rapid and robust vaccine platform. We generated several chimeric SARS-CoV-2 genes and have selected for the most potent DNA vaccine candidate delivered by in vivo electroporation that protects against SARS-CoV-2 disease in animal models. We have now taken this vaccine to phase I clinical testing.
The partners in the consortium have done this before and all know-how for reliable development is present. KI and FoHM developed the vaccine candidates and infectious models, JLU tested candidates for over-activation of innate immunity, IGEA provided a CE marked device for in vivo electroporation in humans. Northx have produced HQ plasmid for the toxicological studies and GMP plasmid for the phase I clinical study, Adlego/Scantox has performed toxicological testing according to GLP, and Karolinska wrote the IB (Investigator’s brochure) and IMPD (Investigational Medicinal Products Dossier), and obtained ethics committee and EMA. A phase I clinical trial of the vaccine in healthy volunteers is ongoing.
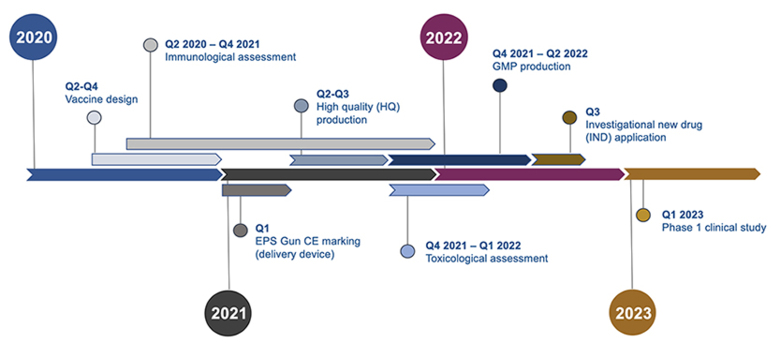
Timeline
OpenCorona in media
Vaccin Insights 13 June, 2023
OPENCORONA: lessons learned from a pandemic vaccine consortium.
LäkemedelsVärlden 6 August, 2022:
Test på människa närmar sig för svenskt vaccin.
Expressen 29 Nov, 2020:
Här skapas vaccinet som ska klara framtida pandemier.
Dagens Nyheter 25 June, 2020:
Svenska vaccinförsöken i ny fas: ”Det är nu det spännande börjar”.
Forskning.se 31 March, 2020:
Vaccinutveckling mot coronaviruset går in i nästa fas.
Dagens Nyheter 7 March, 2020:
Därför dröjer vaccin mot nya coronaviruset.
Pressrelease
12 June, 2023.
Covid-19 vaccine that can cope with mutated viruses in clinical phase 1 study at Karolinska University Hospital.
Partners
KAROLINSKA INSTITUTET (KI)
Prof. DDS. PhD Matti Sällberg
JUSTUS-LIEBIG-UNIVERSITAET GIESSEN (JLU)
Prof. Dr. Friedman Weber
FOLKHALSOMYNDIGHETEN (FoHM)
Prof. Ali Mirazimi
IGEA SPA (IGEA)
Dr Matteo Cadossi
NORTHX BIOLOGICS
Site Manager Lars Fahlander
ADLEGO BIOMEDICAL AB (Adlego)
Dr Urban Höglund
REGION STOCKHOLM (Karolinska)
Katja Tobin, Soo Aleman
Facts
Title: OPENCORONA
Project number: 101003666
Topic: SC1-PHE-CORONAVIRUS-2020
Budget: 3 000 000€
More information about Opencorona.
Publications:
The SARS-CoV-2 N Protein Is a Good Component in a Vaccine.
Ahlén G, Frelin L, Nikouyan N, Weber F, Höglund U, Larsson O, Westman M, Tuvesson O, Gidlund EK, Cadossi M, Appelberg S, Mirazimi A, Sällberg M,
J Virol 2020 Aug;94(18):
Imaging of SARS-CoV-2 infected Vero E6 cells by helium ion microscopy.
Frese N, Schmerer P, Wortmann M, Schürmann M, König M, Westphal M, Weber F, Sudhoff H, Gölzhäuser A
Beilstein J Nanotechnol 2021 ;12():172-179
Inhibition of SARS-CoV-2 by type I and type III interferons.
Felgenhauer U, Schoen A, Gad HH, Hartmann R, Schaubmar AR, Failing K, Drosten C, Weber F
J Biol Chem 2020 Oct;295(41):13958-13964
A universal SARS-CoV DNA vaccine inducing highly cross-reactive neutralizing antibodies and T cells.
Appelberg S, Ahlén G, Yan J, Nikouyan N, Weber S, Larsson O, Höglund U, Aleman S, Weber F, Perlhamre E, Apro J, Gidlund EK, Tuvesson O, Salati S, Cadossi M, Tegel H, Hober S, Frelin L, Mirazimi A, Sällberg M
EMBO Mol Med 2022 Oct;14(10):e15821
(Raw data to EMBO Mol Med paper - Figure 1-3)
Questions about the project
If you have any questions or inquiries about the OPENCORONA project please send an email to Project Coordinator Matti Sällberg.
Matti Sällberg
Project CoordinatorPublic project deliverables
This project is sponsored by the European Union.

