Project 1: Program in Cell Signaling
Our laboratory is studying cellular differentiation and the way this process is regulated by signal transduction.
The main focus is on a cytoplasmic protein-tyrosine kinase (PTK) designated Bruton’s tyrosine kinase, BTK. BTK belongs to the second largest family of mammalian PTKs, the TEC family (Smith et al., 2001) comprising BTK, BMX (ETK), ITK, TEC and TXK (RLK). BTK is affected in the disease named X-linked agammaglobulinemia (XLA), and was identified as a result of positional cloning (Vetrie et al., 1993). BTK was found to shuttle between the cytoplasm and the nucleus (Mohamed et al., 2000). BTK activates NF-kB signaling and is also regulated by this pathway through its own promoter (Yu et al. 2008).
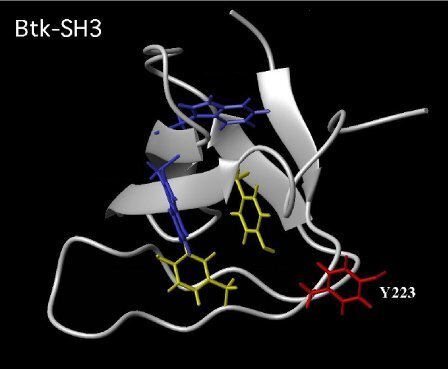
One approach to understand the signaling process is to investigate structure-function relationships (Hansson et al., 1998 Fig. 1; Márquez et al., 2003). Main activities include cell biology, biochemistry, proteomics, imaging, expression profiling, and siRNA technology. In expression profiling studies of the ITK kinase, a unique transcriptome was identified, including the transcription factor PLZF (Blomberg et al., 2009; Raberger et al. 2008). These studies also include research in Drosophila (Hamada-Kawaguchi et al., 2014).
Using proteomics approaches, we have recently identified ankyrin repeat domain protein 54 (ANK54) and 14-3-3z as partners, which regulate BTK nucleo-cytoplasmic shuttling and degradation (Gustafsson et al. 2012, Mohammad et al. 2013). We are currently also studying other proteins, which show functional interaction with BTK, such as AKT.
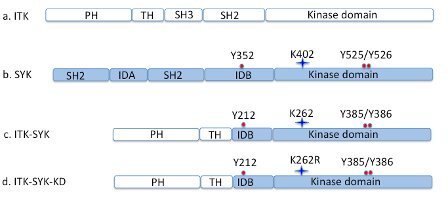
Recently the translocation-based fusion-protein ITK-SYK causing lymphomas in humans has been investigated (Hussain et al., 2009 and 2013, Fig. 3). Studies related to BTK inhibitors, especially ibrutinib, are also ongoing with a focus on leukemia and lymphoma treatment.
We are also developing new treatment modalities for XLA based on splice-correcting oligonucleotides. Here we have generated humanized, transgenic mice lacking mouse BTK but carrying a splicing-deficient human BTK mutant. We have successfully corrected BTK pre-mRNA in B cells from these mice and restored BTK function in vitro (Bestas et al. manuscript).

Selected references
Transcriptional signatures of Itk-deficient CD3+, CD4+ and CD8+ T-cells.
Blomberg KE, Boucheron N, Lindvall JM, Yu L, Raberger J, Berglöf A, et al
BMC Genomics 2009 May;10():233
Regulation of nucleocytoplasmic shuttling of Bruton's tyrosine kinase (Btk) through a novel SH3-dependent interaction with ankyrin repeat domain 54 (ANKRD54).
Gustafsson MO, Hussain A, Mohammad DK, Mohamed AJ, Nguyen V, Metalnikov P, et al
Mol Cell Biol 2012 Jul;32(13):2440-53
Btk29A promotes Wnt4 signaling in the niche to terminate germ cell proliferation in Drosophila.
Hamada-Kawaguchi N, Nore BF, Kuwada Y, Smith CI, Yamamoto D
Science 2014 Jan;343(6168):294-7
Silencing of Bruton's tyrosine kinase (Btk) using short interfering RNA duplexes (siRNA).
Heinonen JE, Smith CI, Nore BF
FEBS Lett 2002 Sep;527(1-3):274-8
Phosphatidylinositol-3-kinase-dependent phosphorylation of SLP-76 by the lymphoma-associated ITK-SYK fusion-protein.
Hussain A, Faryal R, Nore BF, Mohamed AJ, Smith CI
Biochem Biophys Res Commun 2009 Dec;390(3):892-6
Signaling of the ITK (interleukin 2-inducible T cell kinase)-SYK (spleen tyrosine kinase) fusion kinase is dependent on adapter SLP-76 and on the adapter function of the kinases SYK and ZAP70.
Hussain A, Mohammad DK, Gustafsson MO, Uslu M, Hamasy A, Nore BF, et al
J Biol Chem 2013 Mar;288(10):7338-50
Bruton's tyrosine kinase (Btk): function, regulation, and transformation with special emphasis on the PH domain.
Mohamed AJ, Yu L, Bäckesjö CM, Vargas L, Faryal R, Aints A, et al
Immunol Rev 2009 Mar;228(1):58-73
Regulation of nucleocytoplasmic shuttling of Bruton's tyrosine kinase (Btk) through a novel SH3-dependent interaction with ankyrin repeat domain 54 (ANKRD54).
Gustafsson MO, Hussain A, Mohammad DK, Mohamed AJ, Nguyen V, Metalnikov P, et al
Mol Cell Biol 2012 Jul;32(13):2440-53
Dual phosphorylation of Btk by Akt/protein kinase b provides docking for 14-3-3ζ, regulates shuttling, and attenuates both tonic and induced signaling in B cells.
Mohammad DK, Nore BF, Hussain A, Gustafsson MO, Mohamed AJ, Smith CI
Mol Cell Biol 2013 Aug;33(16):3214-26
Differential evolutionary wiring of the tyrosine kinase Btk.
Nawaz HM, Kylsten P, Hamada N, Yamamoto D, Smith CI, Lindvall JM
PLoS One 2012 ;7(5):e35640
The transcriptional regulator PLZF induces the development of CD44 high memory phenotype T cells.
Raberger J, Schebesta A, Sakaguchi S, Boucheron N, Blomberg KE, Berglöf A, et al
Proc Natl Acad Sci U S A 2008 Nov;105(46):17919-24
Single-stranded DNA oligonucleotides inhibit TLR3-mediated responses in human monocyte-derived dendritic cells and in vivo in cynomolgus macaques.
Sköld AE, Hasan M, Vargas L, Saidi H, Bosquet N, Le Grand R, et al
Blood 2012 Jul;120(4):768-77
The Tec family of cytoplasmic tyrosine kinases: mammalian Btk, Bmx, Itk, Tec, Txk and homologs in other species.
Smith CI, Islam TC, Mattsson PT, Mohamed AJ, Nore BF, Vihinen M
Bioessays 2001 May;23(5):436-46
The gene involved in X-linked agammaglobulinaemia is a member of the Src family of protein-tyrosine kinases. 1993.
Vetrie D, Vořechovský I, Sideras P, Holland J, Davies A, Flinter F, et al
J Immunol 2012 Apr;188(7):2948-55
Proteasome-dependent autoregulation of Bruton tyrosine kinase (Btk) promoter via NF-kappaB.
Yu L, Mohamed AJ, Simonson OE, Vargas L, Blomberg KE, Björkstrand B, et al
Blood 2008 May;111(9):4617-26
Regulation of Bruton tyrosine kinase by the peptidylprolyl isomerase Pin1.
Yu L, Mohamed AJ, Vargas L, Berglöf A, Finn G, Lu KP, et al
J Biol Chem 2006 Jun;281(26):18201-7
