The Karolinska Institute Small Molecule Mass Spectrometry Core Facility (KI-SMMS)
A new resource at KI and Region Stockholm to provide custom analyses of small molecules.

Following extensive investment in custom-built facilities, the Small Molecule Mass Spectrometry Core Facility (KI-SMMS), is operating at full speed as of Fall 2023. This core facility is hosted by the Institute of Environmental Medicine (IMM) and located at Biomedicum. It is equipped with an array of mass spectrometry systems and commensurate sample preparation equipment tailored to the specific needs of KI and Region Stockholm researchers. Antonio Checa Gómez, Senior Research Infrastructure Specialist managing the SMMS-CF, discusses the design and organization of the core facility as well as provides insights into the future directions of the facility.
What is mass spectrometry?
The core facility uses a technology called mass spectrometry to quantify small molecules in biological samples. Mass spectrometry is an analytical technique that measures the mass-to-charge ratio (m/z) of charged molecules present in a sample. Chemicals are identified by the sorting of gaseous ions in electric fields according to their mass-to-charge ratios. The mass spectrum can be used to determine the mass of a molecule, its elemental and isotopic composition, or to elucidate its chemical structure. The Karolinska has a long history of development in mass spectrometry, with one of the first gas chromatograph interfaced to a mass spectrometer (GC/MS) instruments designed and built by Ragnar Ryhage, who led the Mass Spectrometry Laboratory at KI from 1950-1981. The importance of the ionization process in mass spectrometry was recognized by awarding the Nobel Prize in Chemistry (2002) to John Fenn and Koichi Tanaka. Mass spectrometry has become the predominant method used in metabolomics and lipidomics. Here in the KI-SMMS facility, we strive to continue the tradition of mass spectrometry at KI and have developed a state-of-the-art instrument park dedicated to serving the current and future research needs of KI and Region Stockholm.
What services do you currently offer?
Our core facility provides an array of methods to quantify small molecules in all sample types. Our main goal is the generation of high-quality mass-spectrometry data that fulfils the specific needs of each user. With that in mind, we assist our users in all parts of the analysis pipeline, from experimental design to interpretation of the data.
Our standard methods include a series of state-of-the-art panels covering different pathways within the metabolome and lipidome. These include broad profiling metabolomics and lipidomics methods capable of simultaneously measuring hundreds of compounds as well as targeted methods for specific questions (e.g., TCA cycle, amino acids). We also offer specialized lipid mediator methods, such as eicosanoid and sphingolipid panels, that we have developed over many years and have been used in multiple projects by both academic and industrial researchers as well as in clinical trials.
A particular advantage of our facility is that we also develop custom methods for small molecule analyses not included in our standard panels. This is necessary for cases in which users come with challenging sample matrices or for compounds that are at very low concentrations and require specific sample processing methods. We pride ourselves on being able to offer these custom services to the academic community that would often be cost prohibitive in contract research organizations (CROs).
How can researchers and other users benefit from these methodologies?
While our standard panels focus on a series of methods with broad coverage of biochemistry, the number of small molecules that can be measured using mass spectrometry is endless, and so are the possibilities for our users. Estimates of the human metabolome currently run at hundreds of thousands to millions of molecules. Clearly, it is not possible to analyze all of these compounds in a single experiment. We therefore work to tailor our methods to the unique molecular needs of our users. This approach synergizes well with large research institutions like KI, with researchers working in a breadth of fields and applications for which we need to target and measure thousands of potential compounds.
The molecular variety is very diverse, requiring multiple methods and broad expertise. As examples, we are able to measure amino acids and derivatives, nucleosides, TCA metabolites, sugars, carnitines, ceramides, and phosphatidylcholines, just to name a few. As long as they are present, all of these compounds can be measured in a few microliters of sample, at the same time, in virtually any matrix one can think of. Given the advances in mass spectrometry, it has become standard practice to use these broad profiling methods. One of our tasks is now to efficiently communicate this possibility to the research community. These advanced methods can open up many novel experimental strategies for KI researchers.
What are the main challenges?
While the methods are broadly applicable, there are nuances related to the specific matrix in which they are applied. For instance, when trying to profile the optic nerve of a mouse, there are only a few micrograms of tissue available. This is hard to see and challenging to manipulate, and therefore requires specific method development. Ensuring that the results are comparable between samples is a priority for our work, which while sometimes challenging, is vital for data quality.
The other main challenge is related to the large number of potential applications. For example, in a standard CRO or a clinical laboratory, one can receive a large number of samples, but they will be in the same format (i.e., same matrix, same tube), to be analyzed with the same method(s), with a predictable number of samples received in a given period of time. However, in our facility, we often receive a handful of samples in a non-standard collection format from a range of sample matrices to measure different molecules. These cases are not compatible with standardized workflows, which results in decreased throughput and increased costs. Fortunately, the support received by KI/SLL enables us to partially subsidize these costs for researchers at Swedish institutions.
What is next in your pipeline?
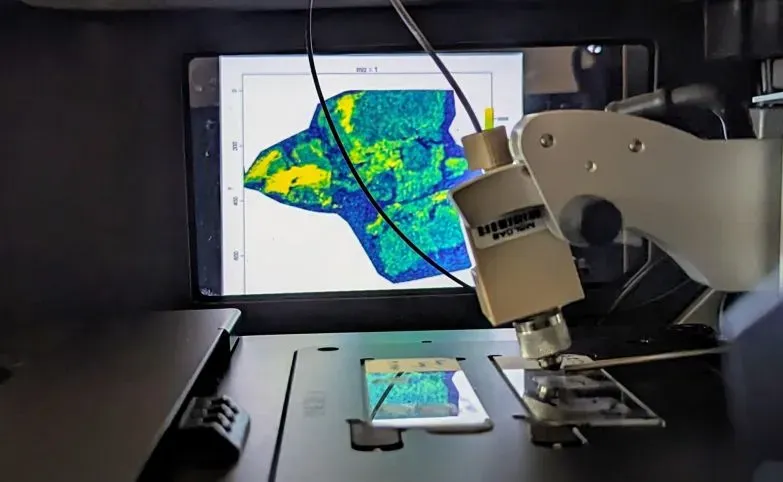
The core is co-located in the Unit of Integrative Metabolomics at IMM together with the research group of Craig Wheelock, the Scientific Director. This enables us to work in parallel in the development of new MS-based methodologies, which we can then make available through the core facility.
For instance, we are now working on the establishment of ambient mass spectrometry-based applications. This opens a vast field of potential uses, from truly high-throughput screening of metabolites in non-complex matrices to mass spectrometry imaging of tissues. The Wheelock lab has recently acquired the first instrument on the market that combines DESI, an ambient mass spectrometry ionization source, with the Xevo TQ Absolute, the most sensitive mass spectrometer from Waters. The advantage of this instrument is its fast scan rate combined with very low limits of detection. This will allow researchers to study, for instance, how a drug or a metabolite distributes within a tissue. The proximity of Waters in the Innomedicum facilities on the Solna Campus enables close collaborations to establish cutting-edge methods in the KI-SMMS. For example, the first images have already been acquired in the Wheelock lab and our objective is to make this method available as a service for KI users in the beginning of 2025. Further developments are planned to increase the spatial resolution of our imaging methods to enable single cell imaging in the near future.