ARG - our offer
The Autoradiography core facility (ARG CF) is fully outfitted and staffed to support autoradiography (in vitro and ex vivo) and radioligand binding assays (whole cells or tissue homogenates) for different types of projects, e.g. target-specificity, binding affinity, drug screening.
We provide full service, including consultation, experimental design, tissue sectioning, assay execution, analysis and reporting. You are always welcome to get in touch with us and discuss a project.
We provide training and access to equipment for our users to carry out their studies on their own, booking lab space and instruments via our iLab page to do so.
Many of our services are offered in synergy with other facilities in the center for imaging research (CIR). The combined services and infrastructure offered by CIR give users the opportunity to develop fully translational molecular imaging studies ranging from drug screening and radioligand development and production to translational in vivo and clinical PET and MRI imaging.
We work closely with the Radiopharmacy (RCF), the pre-clinical and clinical imaging facilities (KERIC & BMIC).
We have extensive experience with academic projects and academic-pharmaceutical industry partnership projects and can give you the highest quality service.
Services
Our services are commonly used in drug or PET tracer development, for example in the evaluation of radioligands for misfolded proteins, such as tau and alpha-synuclein. We also run studies in theranostic tracer development projects.
We perform [35S]GTPγS assays of GPCR activity. Our autoradiography service includes tritiated ligands as well as PET radioligands labelled with short-lived isotopes (e.g. 18F, 11C).
We can help you to
- Find new drug candidates, screening for compounds that compete with high affinity for binding to a particular target.
- Visualize and quantify radioligand localization in different anatomical regions / biodistribution.
- Assess target-specificity of a candidate drug or PET tracer.
- Characterize the binding kinetics and pharmacodynamic properties of a candidate drug/compound for a given target.
- Measure potency and efficacy of a drug candidate for a GPCR.
- Characterize phenotype differences of GPCR activity in your model of choice.
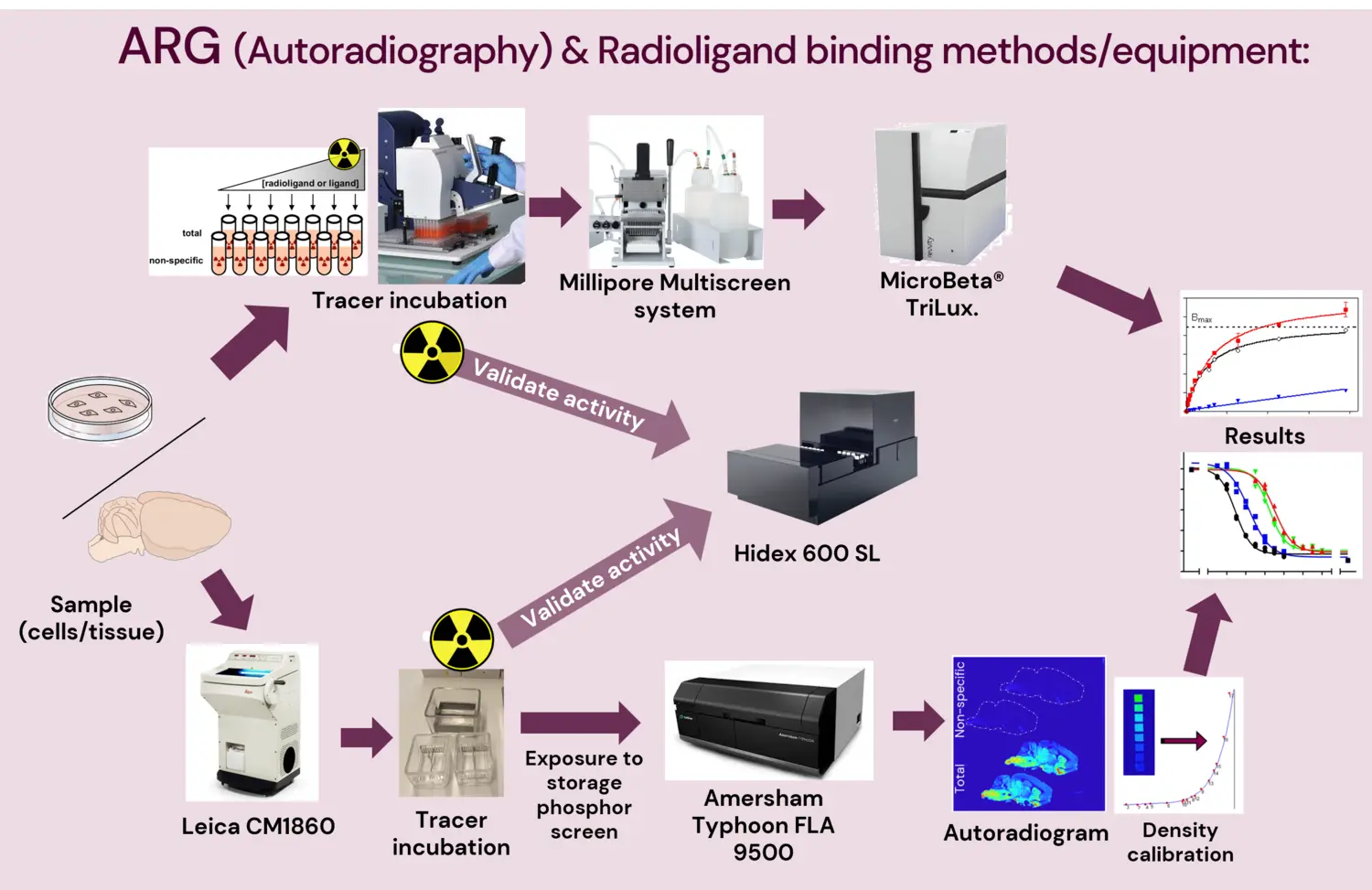
Methods offered
Autoradiography
Autoradiography is a quantitative in vitro or ex vivo method employed to visualize and quantify the binding of radioactive isotope-labelled compounds (normally called “radioligand” or “tracer”) to specific targets (e.g. receptors, transporters, enzymes, protein aggregates) in post-mortem or excised tissue from human donors or animal models. [Whole body autoradiography is only currently possible with mouse models.]
This method is a critical component in drug development, for initial characterization of a compound’s biodistribution, binding kinetics and pharmacodynamic properties to predict the suitability of its application in vivo. It is also a useful tool for validation of novel radioligands for PET or theranostics since it provides high-resolution images that help characterize the region and target specificity of the tracer.
Besides traditional radioligands, autoradiography can also be done with radiolabeled monoclonal antibodies or other bioconjugates. The use of such radiolabeled bioconjugates in PET and theranostics studies has seen a surge in recent years, particularly in development of next-generation cancer therapies and precision medicine.
The types of autoradiography assays that can be done are largely the same as those which can be done in tissue or cell homogenates (see radioligand binding section below), with the advantage of being able to visualize the anatomical location of the binding in situ, and the disadvantage that using tissue sections limits the throughput of the experimental design and larger volumes of tracer are required.
For validation of tracer specificity, the radioligand binding signal can be compared with immunohistochemical staining of the target protein.
Autoradiograms obtained by exposure and scanning of phosphor storage imaging plates reach a pixel resolution of 50 µm. Higher resolution (<10 µm) can be achieved by applying a thin coat of nuclear emulsion followed by exposure for subsequent examination using dark-field microscopy, where white silver grains represent the bound radioligand. The crystal diameter of the emulsion is smaller than that used to coat the phosphor storage imaging plates. The drawback with emulsion autoradiography is the higher cost and lower throughput.
Radioligand binding
Radioligand binding is a highly accurate, quantitative, and high-throughput method used to study a range of pharmacological properties (binding kinetics and pharmacodynamics) of a radioligand or tracer to a specific target protein. This method uses tissue or cultured cell homogenates, normalized to a defined protein concentration per reaction, which helps reach very high reproducibility and quantitative value.
There are several types of radioligand binding assays that can done, e.g.:
- Saturation binding assays, where samples are incubated, to equilibrium, with increasing concentrations of radioligand to determine of the maximum density of target available to the radioligand (Bmax) and the affinity (i.e. the equilibrium dissociation constant, Kd). Specificity is measured by adding a high concentration of non-labeled compound that is expected to fully displace the radioligand.
- Competition (displacement) binding assays, where samples are co-incubated, to equilibrium, with a fixed concentration of a target-specific radioligand and increasing concentrations of other unlabeled compounds expected to compete for binding to the same target. These assays are mainly used for:
- High throughput screening of (up to hundreds) of compounds for characterizing their affinity to the target.
- Confirming the pharmacological binding site of a candidate tracer / radioligand. This is done by running displacement assays using known drugs (unlabeled) whose specificity and affinity for the target of interest are already known. If these known drugs displace the radioligand, we can determine whether the radioligand specifically binds to that target.
- Binding Kinetics assays, to measure the ligand association (Kon) and dissociation (Koff) rates on a specific target:
- With association binding assays, a fixed concentration of radioligand is added and specific binding is measured at increasing incubation times. This is useful to characterize the interaction of the ligand with the receptor and measure the time it takes for the radioligand to reach binding equilibrium with the target.
- With dissociation binding assays, the radioligand is first allowed to bind the target to equilibrium, then dissociated either by adding a very high concentration of unlabeled competitor or by adding a very large volume of buffer to dilute the concentration of the radioligand to an extremely low and negligible concentration.
- Functional [35S]GTPγS binding assays: If you are studying G protein coupled receptors (GPCRs), the level of G protein activation following agonist binding can be measured with this assay.
- When occupied by an agonist, GPCRs become activated and directly bind to heterotrimeric Gαβγ proteins, which initiates the guanine nucleotide exchange on the Gα subunit, where the dissociation of GDP from Gα allows the binding of GTP in its place. GTP binding leads to the dissociation of the G protein from the receptor and of Gα-GTP from Gβγ, each of which can go on to activate downstream effectors such as e.g. adenylyl cyclase, phospholipase C, and ion channels. Finally, GTPase hydrolysis of GTP to GDP induces the binding of Gα-GDP back to Gβγ, thus inactivating the G protein.
This guanine nucleotide exchange process can be monitored in vitro by measuring the binding of [35S]GTPγS, which is resistant to hydrolysis by GTPase and therefore accumulates in cellular membranes upon GPCR activation. The advantage of this method is that it targets the earliest event in the intracellular signal transduction cascade, so measures are not confounded by downstream signaling amplification or other modulation that other assays can’t exclude.
Studies can be conducted in cell lines and post-mortem tissue samples from animal or human donors. With this assay you can for example:
- Measure potency and efficacy of a drug candidate for a GPCR.
- Characterize phenotype differences of GPCR activity in your model of choice.
- Accurately quantify GPCR desensitization in different conditions, treatments, etc.
Full service, or do it yourself
We give our users the opportunity to choose whether to order a full service; or a consultation request to get assistance with planning and experimental design/analysis; or book training and time with the instrument/resource to run experiments on their own.
A training/introduction with each instrument is required for all first-time users.
Note: Users booking our instruments (except the cryostat) must be licensed by the Karolinskasjukhuset radiation safety officer to work with radioisotopes. Check here for available courses this term.
For those holding a license, radiation safety training must have been completed <5 years to the date of use/experimentation for all users.
Instruments and other resources
Phosphoimager
Amersham Typhoon FLA 9500 IP (and eraser)
A laser scanner for imaging of radioisotope-labeled tissue slices (autoradiography). Pixel resolution of up to 25 μm (NOTE! 635 nm excitation only).
Liquid Scintillation Counter
Hidex 600SL
A high throughput liquid scintillation counter that can detect beta and alpha radiation.
Plate filtering and separation system
Millipore MultiScreen
- This system makes it possible to carry out faster multiple sample radioligand binding assays from sample incubation to scintillation counting in a single 96-well plate, reducing radioactive waste and improving coefficients of variation and reproducibility.
Revvity UNIFILTER-96 CELL HARVESTER
- With this interchangeable vacuum filtration and washing system, you can flexibly use either 96 well or 24 well plates, with filterplates or filtermats.
High-throughput pipetting
Gilson PLATEMASTER P220
When using this 96-channel pipettor, the time it takes to fill 96-well plates is significantly reduced to approximately 10-20 seconds or less. This is critical for high-throughput radioligand binding screening studies.
Microplate scintillation counter
Revvity MicroBeta2 counter for radiometric and luminescence detection
A high-throughput plate scintillation counter with 6 detectors. It can count beta, gamma or luminescent labels on filters, in microplates or in tubes.
Cryostat
Leica CM1860
Other
- Lab bench and Chemical safety hood licensed for isotope work
- Various radiation shielding/safety equipment.
Isotopes licensed
Our laboratory and staff are licensed to work with several short and long-lived radioisotopes for in vitro studies. Please contact us to know more.
User fees
For pricing, or to request a quotation for a project please contact us, or check our iLab booking system.
External use of the core facility
ARG can offer services without a commissioned/contract research or collaborative research agreement, in accordance with the Swedish governmental ordinance (2022:1378) on fees for research infrastructure.
Find out more about external use of KI's core facilities
