BFC - our offer
The BFC Core Facility is a shared resource laboratory that provides researchers with the capability of performing a wide range of flow cytometry experiments.
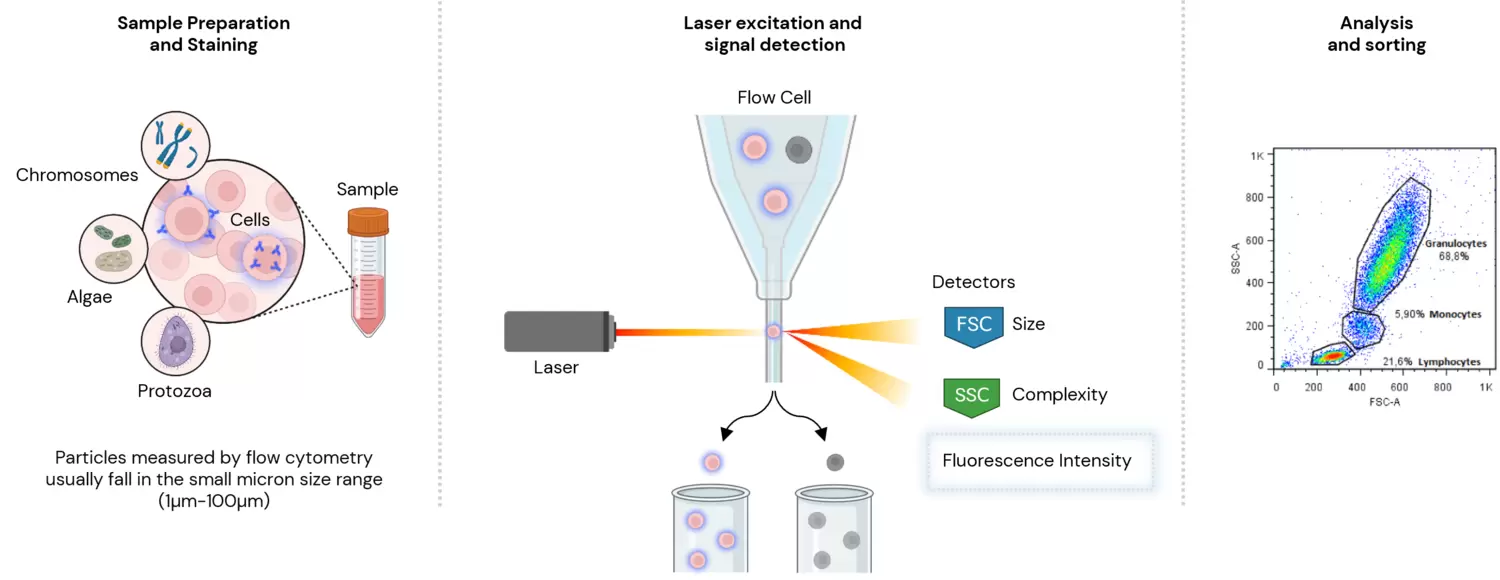
Flow cytometers are traditionally designed for measuring particles, like beads and cells. These tend to fall in the small micron size range. One of the characteristics that we typically measure is the amount of light that the target particle scatter. This we term as either forward or side scatter, based on the position of the detector relative to the excitation light source. However, flow cytometry is predominantly used to measure fluorescence intensity produced by fluorescent-labelled antibodies detecting proteins, or ligands that bind to specific cell-associated molecules. In human whole blood, for example, lymphocytes, monocytes, and granulocytes can be distinguished simply because they scatter laser light differently.
What is Flow cytometry
Flow cytometry is a central technology in biomedical research allowing multiparametric analysis and isolation (by cell sorting) of cell populations down to a single cell from complex cell mixtures.
Flow cytometers utilise lasers and optics to measure the physical and chemical properties of cells and particles in a fluid stream. As the sample flows through a narrow channel, it passes through a laser beam that excites fluorescently labeled cells or particles. The scattered and emitted light is then detected by a series of photomultiplier tubes, which generate electrical signals that are processed by a computer.
By measuring the intensity of scattered and emitted light, flow cytometry can provide information on cell size, granularity, and complexity, as well as the expression of surface and intracellular markers.
The ability to analyse multiple parameters simultaneously enables the identification of rare cells and the characterization of cell populations with high specificity and sensitivity.
Our facility
The core facility provides open access to all interested researchers, regardless of their affiliation, and strives to provide each user with skilled services, education and training to achieve high quality research.
We work with equipment from Becton Dickinson and Sony, Cytek and Beckman Coulter ranging from conventional to full spectral cytometry.
The facility was first established in the 1980s at the Tumor Biology department. It belongs to the department of Microbiology, Tumor and Cell Biology, and is supported by core facility funding from KI/SLL.
To cover running costs, the facility charges fees for services.
Key Services
- Conventional and full spectral cytometry for multi-parametric analysis (self-use and assisted use of analyzers)
- Multiparameter fluorescence activated high speed cell sorting (self-sorting and assisted sorting):
- Full-service sorting of rare populations out of a heterogeneous sample. Cell cloning, particle enrichment, and high purity bulk sorts.
- Temperature controlled collection (cooling or heated collection).
- Eppendorf, tubes and single cell plate deposition.
- Multiple nozzle options to accommodate cells.
- High-recovery and indexed single-cell sorting for sequencing.
- Imaging Flow Cytometry, combining the high throughput capabilities of flow cytometry with cell imaging
- Advice and consultancy on experimental design, sample preparation, data acquisition, analysis and interpretation, as well as technical support and troubleshooting.
- Dedicated training sessions in the use of our flow cytometers, cell sorters and data analysis software
- Data analysis stations with several analysis software: FlowJo, ID7000 Software (Sony), FCS Express (De Novo Software, Dotmatics), IDEAS Software (Cytek Bioscience)
