Childhood Cancer Therapeutic Catalyst
Childhood Cancer Therapeutic Catalyst accelerates the translation of discovery research into novel therapeutics. It seeks projects with strong potential to deliver novel therapeutics for children and young people with cancer. This scheme aims to de-risk early projects to facilitate further drug discovery research, funding, or partnering.
Successful projects will receive up to £250k for up to 18 months.
Cancer Research Horizons is seeking projects with strong potential to deliver novel therapeutics for children and young people with cancer. This scheme aims to de-risk early projects to facilitate further drug discovery research, funding, or partnering.
Eligibility
You should:
- be an academic researcher based at any university or research institute world-wide
- have your own lab
This scheme is open to international applications.
Funding awarded
Successful projects will receive up to £250k for up to 18 months.
These are focussed and milestone-driven awards, therefore go/no-go criteria will be embedded into the project and funding will be awarded through instalments.
Funding can be used to support: staff time (research or technical), associated running expenses and outsourced research activities (where appropriate).
Funded projects may also receive in-kind support and expertise through Therapeutic Innovation.
Deadline
There are two review rounds per year. Deadlines will be posted in the main webpage.
Scientific remit
Cancer Research Horizons invites applications for early drug discovery proposals from across all childhood cancer indications and modalities. Activity that would be considered eligible for support through this award includes:
- deconvolution and validation of novel targets and pathways
- novel therapeutic approaches to validated targets
- development of platforms, assays and screens to identify novel therapeutics
- drug discovery feasibility
Not in remit
The following are examples of activity that are not within the remit of this scheme:
- discovery or basic biological research relating to cancer
- target identification proposals
- full hit finding activities, as likely to be beyond the scope of this award
- preclinical and clinical drug development studies (including, bulk synthesis/manufacture to Good Manufacturing Practice, toxicology to Good Clinical Practice)
- development of technology to address an area of early detection or diagnosis
- exploratory studies to uncover the underpinning mechanisms of resistance to therapies
- novel therapeutic combinations
- repurposing of existing therapeutic agents into a childhood cancer setting
Application Process and Timelines
Before you begin your application consider reaching out to Cancer Research Horizons to discuss your project, its current stage of development and how Cancer Research Horizons might be able to support its progression.
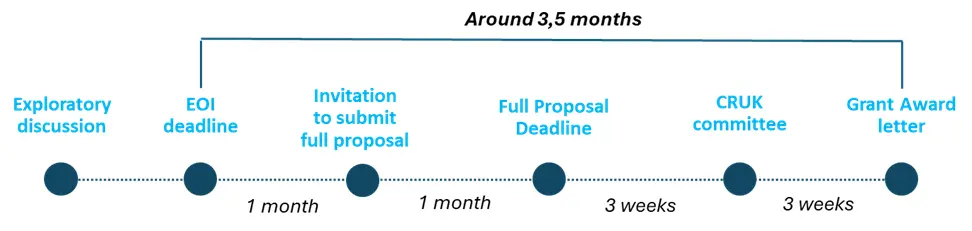
The application process is as follows:
- Expression of interest (EoI)
- Successfully applicants will be invited to submit a full project proposal within a month from the EoI submission.
- Full proposal
- Cancer Research Horizons will arrange a call with successful applicants to discuss the scientific feedback, as well as where Cancer Research Horizons' can provide expertise and capabilities to support your project.
- One of Cancer Research Horizons' scientists will be assigned to work with you to create a collaborative, costed and milestone-driven full project proposal.
- Applicants will be invited to a committee interview within 3 weeks from the full proposal submission and outcomes will be shared within 3 weeks from the interview.
Information on how to apply
FAQ
What makes a successful application?
Successful applications are based on high impact scientific discoveries with a clear route of translation to benefit children and young people with cancer (e.g., robust proof of concept, strong disease positioning hypothesis, innovative models for target validation or identification, etc).
The proposed project must reach an inflection point within the 18-month window, with validation of the hypothesis and positioning of the project for potential onward investment.
Successful applications are fully collaborative in nature, making the most of expertise based in your own research laboratory and our Therapeutic Innovation group.
How is the scheme collaborative?
Funded projects will run collaboratively between academic research groups and Cancer Research Horizons’ drug discovery laboratories (Therapeutic Innovation), with joint decision-making and accountability for project progression.
At the full application stage, you will be assigned a scientist from Therapeutic Innovation to support you in developing your proposal, and during the project if you are successful. These scientists bring significant expertise and serve as a single point of contact to manage the internal (Cancer Research Horizons drug discovery) and external resources required to deliver your project.
Cancer Research Horizons can also offer additional expertise, resources, and capabilities from across Cancer Research Horizons, Cancer Research UK, and our wider investigator network to help your project progress more effectively. Additionally, if appropriate, Cancer Research Horizons can support you in identifying options to accelerate the project toward patient benefit, including through C-Further.
Contact
Contact the Therapeutic Catalyst team, highlighting that you are interested in the Childhood Cancer Therapeutic Catalyst Award, to discuss your proposal.
Later stage projects may be able to access support through our partnered children's and young people's cancer therapeutic consortium, C-Further, where Cancer Research Horizon are a founding partner: https://www.c-further.org/
