Import of mice from non-accredited breeders
Here you will find the most crucial information on how to import mice from non-accredited breeders to Comparative medicine's animal facilities at KI. Depending on your needs, the background of the animal and the barrier level, there are several options available. Our import team will guide you through the process.
Accredited breeders
Accredited breeders are Charles river (Germany, France, Italy), Janvier labs (France), Envigo (Netherlands) and Taconic (Denmark; Tjornberg/Hallingor). For regular import of live animals from accredited breeders please contact your animal facility. Special conditions apply at the KM-W Core breeding in barrier B.
Application to import mice from non-accredited breeders
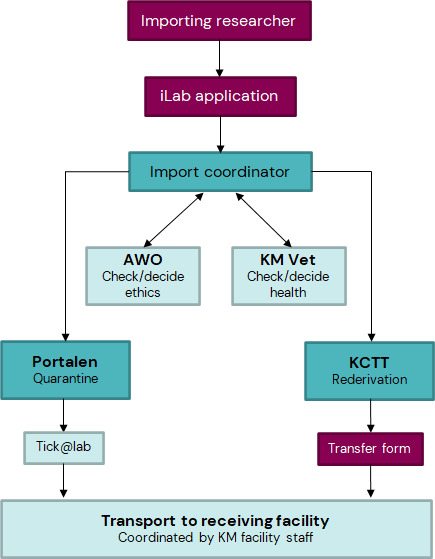
The researcher initiates a new import request via iLab (Import Portal). Please, contact KM import coordinator via import-portal@km.ki.se in case of questions.
The researcher collects and submits all necessary documentation:
- health status of the sending facility (entire barrier) including facility description, health-certificate should not be older than 3 months and covering 18 months back in time
- ethics permit containing the strain
Documents will be reviewed by the Animal Welfare Officer (NACWO) and the facility veterinarian.
A decision is made whether the animals:
- have to be placed into Import Portal (quarantine) for an additional health check (to be specified by receiving facility veterinarian)
OR
- must be re-derived
When importing frozen material or fresh germ cells, the route is the same as for re-derivation. Researchers should initiate an import request via the link above and supply all necessary documents.
Possible options:
Import with recommended quarantine
- Based on the decision of the receiving facility that the mice have to be placed in quarantine, the researcher will be contacted by Import Portal for planning of animal delivery.
- The researcher orders/request the animals and is legally responsible for the transport (researchers name and phone number must be on transport documents).
- The researcher organizes the transport to Import Portal and confirms with the Import portal that the animals can be received on that date and time.
- The manager of Import Portal coordinates the health monitoring.
- The manager of Import Portal informs the researcher and the receiving facility about the health monitoring results.
- The receiving animal facility veterinarian needs to approve the health report and gives the ok to receive the animals (email to researcher and Import Portal).
- The transport to the receiving animal facility is arranged by KM and the researcher is informed when the animals have arrived.
Import with recommended quarantine - as pdf
Import after recommended re-derivation
- Based on the decision of the receiving facility that the mice have to be re-derived, KM import coordinator informs the researcher and KCTT (Karolinska Center for Transgene Technologies) about the upcoming re-derivation and plans the animal delivery with the Import Portal.
- The researcher orders/request the animals and is legally responsible for the transport (researchers name and phone number must be on transport documents) .
- The researcher organizes the transport to Import Portal and confirms with the portal that the animals can be received on that date and time.
- In case of import of frozen or fresh germ cells, the delivery of the material should be coordinated with KCTT .
- KCTT informs the researcher that re-derivation has been done and updates regularly about the results (e.g., number of born pups).
- The animal technicians from the production unit inform the researcher that biopsies for genotyping were taken.
- The health check after re-derivation is done at the production unit (B-barrier).
- The KM import coordinator informs the researcher about the health monitoring results (email the receiving facility veterinarian and the receiving animal facility).
- The receiving animal facility veterinarian needs to approve the health report and gives the ok to receive the animals.
- The researcher fills in the transfer form and sends it to production unit and the receiving animal facility.
- The transport to the receiving animal facility is arranged by KM and the researcher is informed when animals have arrived
Costs associated with imports
Costs of imports are covered by the researcher. The following costs are associated with imports:
- Transport of animals to KI, including transport boxes and documentation
- Cage costs per day at Import portal plus cost of additional services
- Cost of Health monitoring test analysis
- Transport cost to receiving facility
Note that this does not include any costs associated with the exporting facility.
