Clinical Grants 2023-2024
The aim of the SFO Stem cells and Regenerative medicine is to support research and infrastructure within the field of stem cells and regenerative medicine. The SFO supports researchers, research programs and infrastructure at the Karolinska Institutet (KI) with or without collaboration with the health care system based on scope, quality, and the potential to strengthen and develop the field.
The steering committee for SFO stem cells and regenerative medicine has decided to award 2 clinical research grants for 2023 and 2024.
Each grant has a value of 2 million SEK per year.

Stephan Mielke
Towards the first hospital-based treatment platform for sickle cell anemia using gene editing of autologuous blood stem cells
Sickle cell disease (SCD) represents a huge burden to the healthcare system worldwide as curative alternatives are limited to allogeneic stem cell transplantation (SCT). Here, the lack of HLA-matched donors and associated potentially life-threatening alloreactive risks have deprived several patients from this curative option. Recent opportunities with gene editing have raised hope to cure this growing patient group in need. In particular, induction of fetal hemoglobin has been proven to be efficient for treating SCD.
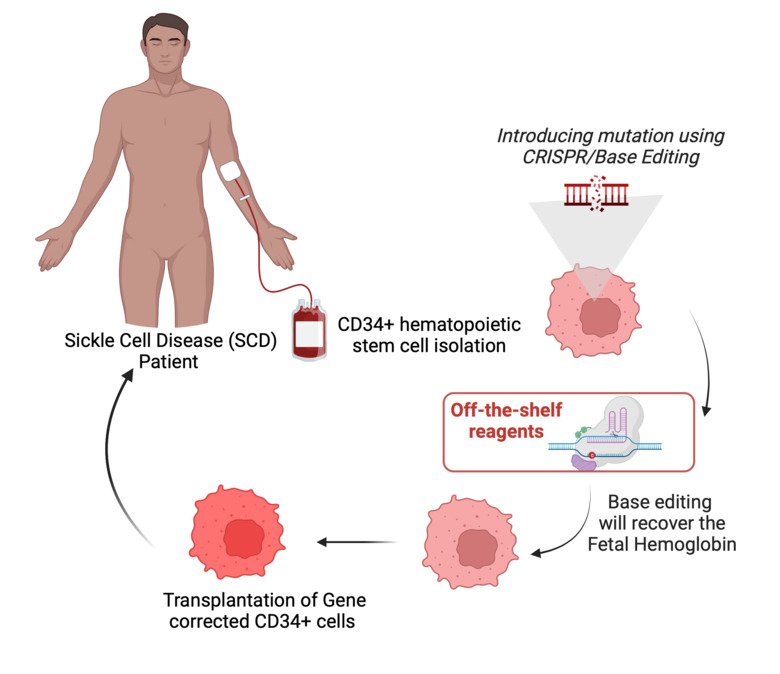
Today, no patient in Sweden has access to gene therapy and sky-rocketing costs make industry-driven manufacturing unlikely in a foreseeable future. We are planning the development of Sweden’s first hospital-based gene editing platform using CRISPR-Cas9 and Base Editing methodologies with the goal to treat the first patient within the three years with gene-edited CD34+ selected autologous SCT.
More research is performed in Stephan Mielke group.

Johanna Ungerstedt
Systemic mastocytosis: deciphering the cellular origin of disease and establishing CCL23 as a diagnostic and prognostic biomarker
Most systemic mastocytosis (SM) patients carry the driver KIT D816V mutation. The majority will have a normal life expectancy however around 10% have a malignant disease with short overall survival. The cellular origin of disease, and what factors determine the clinical fate, are unknown. In addition, there is a lack of disease biomarkers.
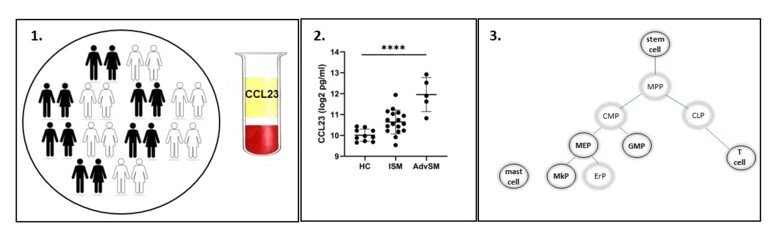
We will establish CCL23 as diagnostic and prognostic disease biomarker and test our hypothesis that the cell of origin of the KIT D816V mutation (stem cell or progenitor) determines the clinical disease course.
Our studies will unravel the cellular origin of SM, establish a new biomarker enabling earlier treatment initiation, improving patient quality of life.
More research is performed in Johanna Ungerstedt group.
