Ethical application for using research animals
In Sweden, animal experiments must be evaluated and approved by a regional ethical committee. An experiment cannot begin until an ethics committee has approved it. It is the research group leader/project director who can apply for ethical evaluation of animal experiments.
At Comparative Medicine's (CM) research animal facilities, a research group leader can apply and hold a research animal ethics license. Hereinafter, this person is referred to as the principal investigator (PI).
Preparations
Prior a principal investigator can initiate a research animal study, the planned study must be approved by a research animal ethics committee (Animal Welfare Act, 7ch., 9-11 §§; Animal Welfare Ordinance 7ch. 6, 8-10 §§; The Swedish board of Agriculture´s regulations and general advice on ethical review of animal experiments (SJVFS 2025:29 sakn nr L152)).
Additional information concerning research animal ethical application, educational requirements and responsibilities are found in The Swedish board of Agriculture´s regulations and general advice on laboratory animal research (SJVFS 2025:28 sakn nr L151).
The principal investigator applies for a research animal ethical review via the e-service “Ansökan om etiskt godkännande av djurförsök” provided by the Swedish Board of Agriculture. All addendums that must be submitted to the research animal ethics committee are also done via this e-service.
The non-technical summary (Populärvetenskapliga sammanfattningen, PVS) for a research animal ethics application or an addendum is done separately in ALURES, an EU-based platform.
Please note! The research animal ethical application and the non-technical summary (NTS) must be written in Swedish.
If you are not already a user of CM:s animal facilities
Please contact the relevant animal facility to discuss the possibilities of using that facility for your studies.
Guides to laboratory animal ethics applications and veterinarian guidelines
You log in to the e-service using an Swedish BankId and to ALURES via your chosen login method.
How to apply – step by step
The Named Animal Welfare Officer (NAWCO) is responsible for questions concering research animal ethics at Comparative Medicine, Karolinska Institutet. For questions concerning research animal ethical applications and licenses: animalethics@km.ki.se
Education
In addition to relevant scientific education and species specific competens, Function B or corresponding competence is required to apply and hold a research animal ethics license. The principal investigator must submit the Function B certificate to the Named Officer for Training and Competence (NTCO) before any research animal ethical application can be considered: NTCO@km.ki.se
General overview for a laboratory animal ethics application
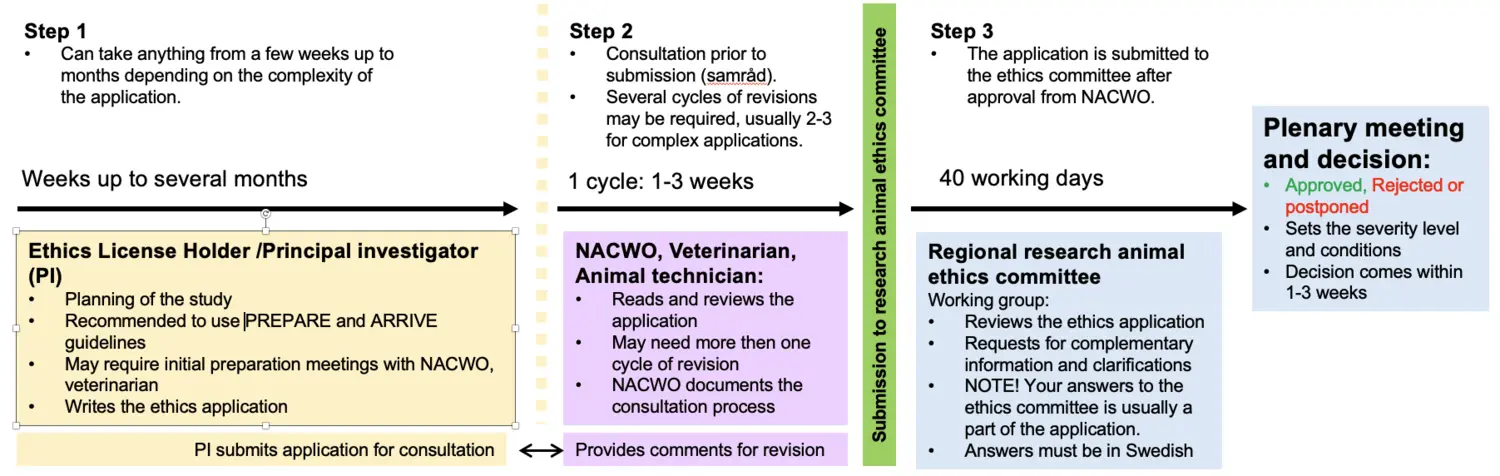
This applies to KM-Annexet, KM-Biomedicum, KM-C, KM-Wallenberg and KM-Fragraeus.
- The principal investigator must be connected to the correct establishment license in the e-service. This is done by the NACWO after that the Function B competence has been validated by the NTCO.
- The principal investigator must initiate the ethical application in the e-service. Please contact the NACWO if additional persons should be added to the application since such persons must be connected to the establishment license.
- It is recommended to carefully plan the application prior the principal investigator add the application to the e-service. It may be motivated to discuss more complex applications with the NACWO at an early stage.
- Consultation according to SJVFS 2025:28 saknr L151: The planning of the application must occur in consultation with NACWO, veterinarian and representative from animal facility/ies involved in the study. The principal investigator downloads PDF-files of the application in e-tjänsten and the non-technical summary in ALURES and submit them to the NACWO. The NACWO coordinates the consultation which can occur via email, phone, or a meeting.
NOTE! Do not submit the non-technical summary to the research animal ethics committee before the NACWO has seen it. Remember to add the id-number (NTS-id) for the non-technical summary obtained from ALURES to the application in the e-service. - The principal investigator revises the application and/or non-technical summary in e-service/Alures according to the recommendations from the consultation. For more complex applications, the consultation process may need to be repeated. If the principal investigator does not consult with prior to submission of the application to the research animal ethics committee, the establishment license holder may deny the principal investigator to perform experiments at the CM animal facilities.
After the last revision, the principal investigator can submit the application and the non-technical summary to the research animal ethics committee via e-service/Alures. This occurs in three steps:
A) The principal investigator submits the application in the e-service and the NACWO reviews and approves the application for submission to the research animal ethics committee.
B) The principal investigator goes into the e-service and sends the application to the committee.
C) The non-technical summary is submitted by the principal investigator via ALURES.
- An application fee must be paid to the Swedish Board of Agriculture. The principal investigator pays the fee at the Swedish Board of Agriculture’s web store. The principal investigator will receive an order that must be added to the application in the e-service.
- The Stockholm research animal ethics committee, once the application has reached them, sends out an information email to the principal investigator. The mail is usually sent within three weeks after arrival, and contains general information and, when applicable, remarks specific to the application.
An applicant who has not received such an e-mail, even though three weeks have passed since submission, should check the status of the application in the e-services. There may be a problem due to a technical issue, or the application may not have been completed. Contact the Swedish board of Agriculture if there are problems with the e-service. The research animal ethics committee has 40 working days to handle your application provided that the application is complete. The application is reviewed by a working group that will, if necessary, contact the principal investigator with required complementary information and questions. A decision on an application can be delayed or rejected if the principal investigator does not answer the work group's request in time.
The decision on the application will be sent to the principal investigator ca 1-3 weeks after the research animal ethics committee plenary meeting. The principal investigator will be contacted by the working group with further questions and complementary information if the application has been deferred.
Please note:
- The principal investigator can apply for an exemption from the Swedish Board of Agriculture if the principal investigator lacks a Swedish BankID.
- Please contact the NACWO if facilities, e.g. AKM/KERIC, Stockholm University, not included in the KM establishment license will be added to the application.
- The decision off the research animal ethical approval can be requested as a public document according to Swedish legislation. The non-technical summary is published anonymously in DECLARE/ALURES within six months after the decision.
After approval of your research animal ethical application
The principal investigator needs to take below actions once the decision of the new animal ethical approval has been received:
Control the decision
Carefully read through the entire decision since there may be conditions or changes in severity levels set by the research animal ethics committee. The principal investigator can appeal to the Central Laboratory Animal Ethics Committee (CDFN). This must be done within three weeks of the receipt of the decision.
Please note that the questions and answers usually is part of the decision.
The principal investigator must submit to a retroactive evaluation if the research animal ethics license is classified as "severe" ("avsevärd svårhetsgrad"). This is done after the research animal ethical approval has expired and is coordinated by the CDFN. Retroactive evaluation may be requested by the research animal ethics committee also for ethics licenses with other severity levels.
2. Archiving
The principal investigator must archive the research animal ethical approval according to the KI's policy document for archiving.
3. Second consultation
Prior to or in connection with project start under a research animal ethics license, obligatory for all approvals deemed severe (Avsevärd svårhetsgrad). The NACWO coordinates the meeting where veterinarian, animal technician and principal investigator's research group are included.
This post-approval meeting can be short and concise but may also require follow-up meetings and the purpose is to facilitate communication and that all required documents e.g. project plan/s, risk assessments etc are in place. For more information: KM Facilities
Addendums to valid research animal ethical approvals
Research animal ethical approvals may need addendums with time. An addendum is done in SJV's e-service, the non-technical summary in ALURES following consultation with NACWO as described above. For tips, advice, veterinarian plans etc, please login at KM Facilities
All administrative changes, as change of principal investigator or addition of a CM animal facility is done via the local Animal Welfare Body (AWB). Addendums without any risks of negative effects on animal welfare can be submitted to the AWB. You find more information and the application form at the Animal welfare body.
