Molecular pain research
Chronic pain is a major health problem affecting about 20 per cent of the Swedish population, resulting in markedly reduced quality of life for the individual.
Pain is also a substantial socio-economical problem and the cost for chronic pain in Sweden has been estimated to a staggering 87.5 billion SEK in medical treatment, loss of productivity and disability (SBU, Swedish Council on Health Technology Assessment, 2006). Unfortunately, there are currently few available effective treatments for chronic pain conditions. Hence, we believe that it is critical to increase our understanding for how chronic pain is regulated in order to identify new treatment strategies and novel drug targets for pain relief.
We are currently studying pain in pathological conditions, such as rheumatoid arthritis (RA), osteoarthritis (OA), fibromyalgia and low back pain, and we are working together with both preclinical and clinical researchers with a mission to increase our ability to study pain from a translational aspect from bench to bedside.
Research Projects
The research in our laboratory is centered on mechanisms that regulate pain signaling, with a particular focus on exploring the role of autoantibodies, nerve growth factor (NGF) and pro/anti inflammatory factors in pain signal transmission in both peripheral and central nervous system. We are currently using several experimental pain models and clinical data/tissues from patient cohorts with the aim to pin point novel targets for the development of new pain therapeutics.
Autoantibody-mediated pain mechanism
Anti–citrullinated protein antibodies (ACPA)
ACPA are autoantibodies that are associated with worse RA disease outcomes and used for diagnosis in RA. Similar to pain, ACPA are often present years before RA diagnosis and the onset of joint inflammation.
Working with a multidisciplinary team of rheumatologists, immunologists and neuroscientists we aim to better understand how ACPA drives pain in the absence of overt joint inflammation. We have previously found that transferring purified ACPA from RA patients into experimental model promotes the development of pain-like behavior, but interestingly visual signs of joint inflammation did not develop. Now, with improved technologies, our team is able to isolate and clone ACPA producing cells from RA patients.
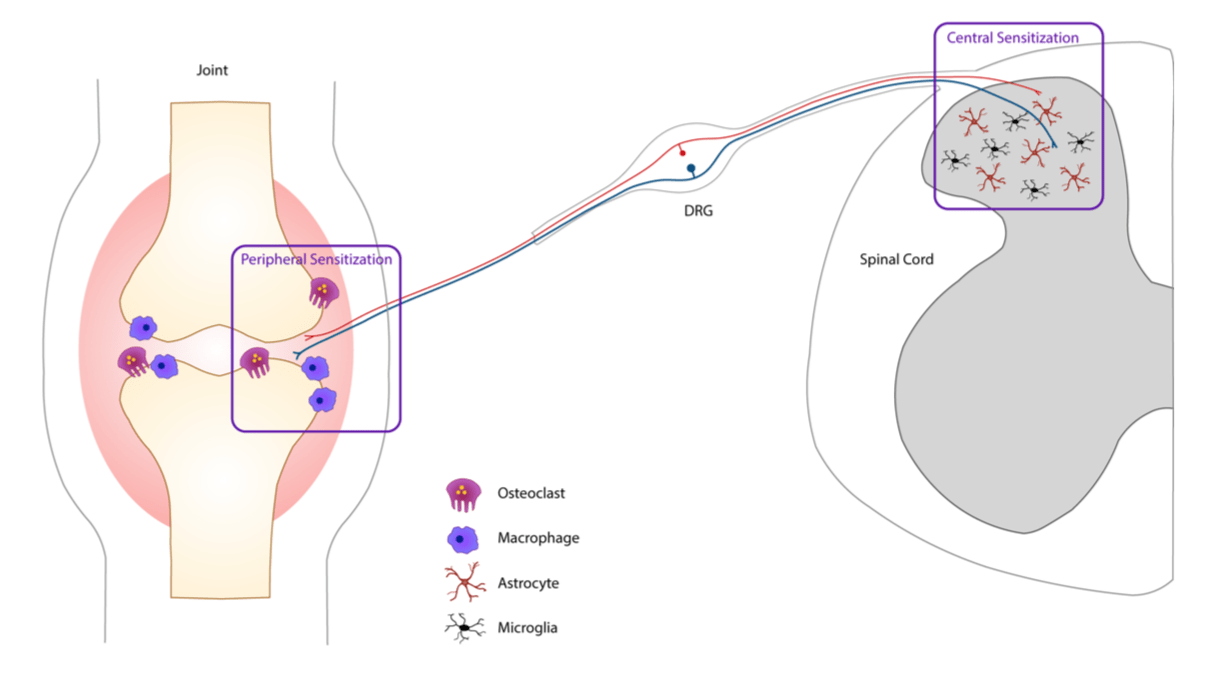
So far, our research has found ACPA-mediated pain involves a fascinating interplay between the immune system, the nervous system and bone homeostasis. We are currently characterizing different cell types contributing to ACPA-driven pain such as osteoclasts, macrophages, microglia and astrocytes.
Collagen antibody-induced arthritis (CAIA)
The CAIA model is commonly used to study the disease mechanism in RA that prior to our studies have not been used for assessment of arthritis-induced nociception.
We have shown this model develops pain-like behavior before signs of inflammation, during inflammation and after inflammation resolves, similarly to the clinical situations. Using behavioral, electrophysiological, molecular and imaging techniques and genetically modified tools, we are currently focusing on studying the mechanisms underlying pain uncoupled from inflammation that we observe before and after inflammatory phase of the CAIA model.
Early CAIA
We are investigating a novel pain mechanism in RA in which autoantibodies could act as pain-inducing molecules independent of the inflammatory process.
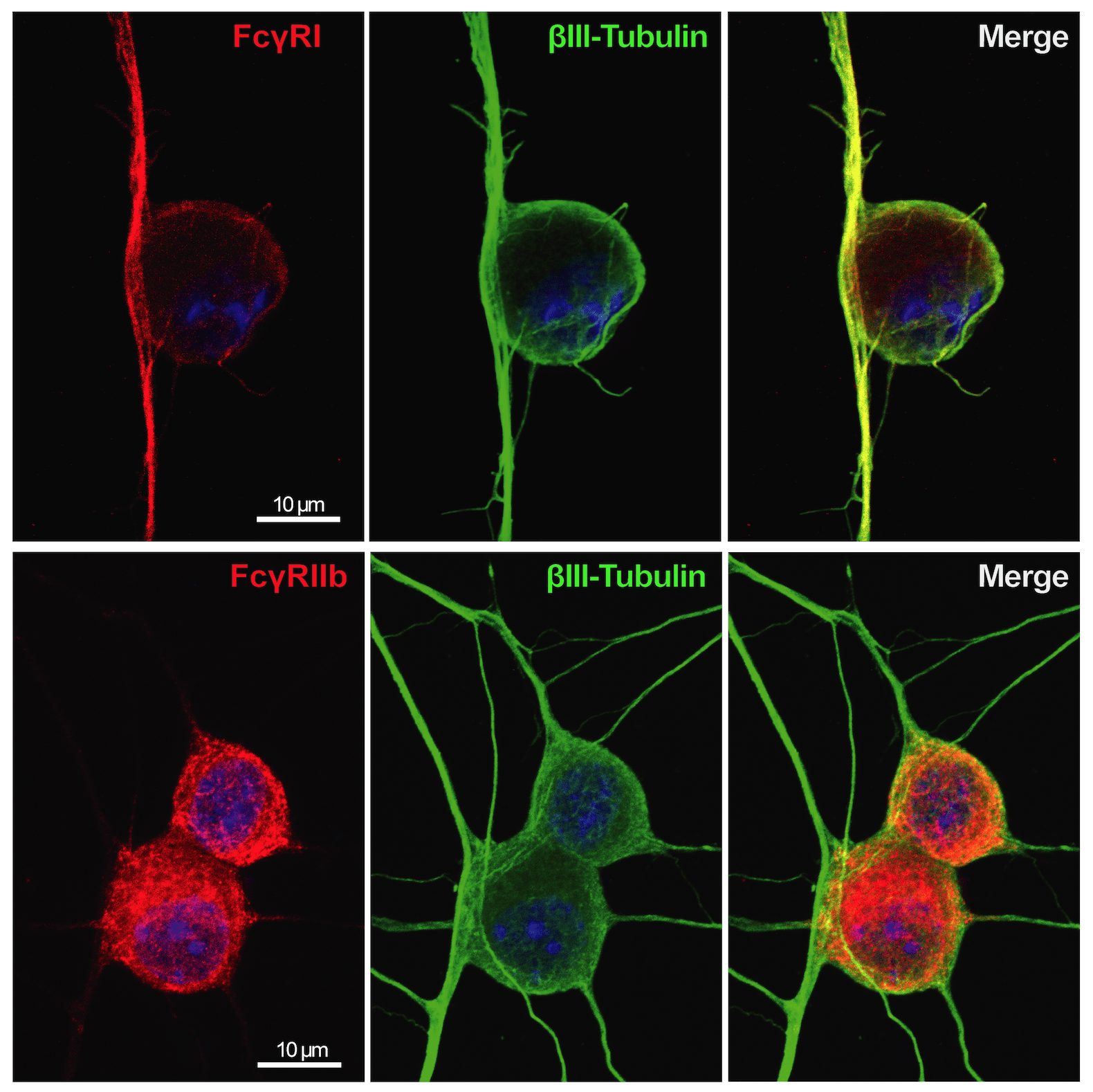
The expression of Fc gamma receptors (I and IIb) was detected in sensory neurons. These receptors can bind autoantibodies in immune-complex formation and therefore sensory neurons can be directly activated without the involvement of any inflammatory cells, which is responsible for the early pain-like behavior we see in the CAIA model.
This project has also a strong translational potential to the human setting since we discovered the presence of Fc gamma receptor III on human adult DRGs, which could open new avenues for development of pain treatments not only for RA, but also for other autoimmune diseases, which present deposits of immune complexes in innervated tissues.
Late CAIA
Another striking observation we made in the CAIA model was that not only pain was persistent, bone destruction was also present post-inflammation.
Through collaborations with bone biologists and rheumatologists, our current work aims to delineate changes in bone metabolism and how that may contribute to chronic pain. In this project, we are exploring the question if bone erosion, vascularization and innervation regulate late phase CAIA-induced pain-like behavior. In addition, we are examining potential factors driving bone erosion and/or pain signal transmission, such as HMGB1 and LPA, which have been associated with RA pathology.
Fibromyalgia
Fibromyalgia is a common rheumatological disorder in which patients are affected by chronic widespread pain in musculoskeletal tissues.
This project focuses on investigating mechanisms by which autoantibodies from FM patients could be directly implicated in nociception. We purified IgGs from FM patients or healthy controls and are currently examining if their transfer to an experimental model would induce pain-like behavior and histological or molecular changes both in the central and peripheral nervous system
Role of NGF in RA-induced pain
NGF has been implicated in joint pain and interestingly a missense mutation in the Ngf gene that has been identified in a Norrbottnian Swedish family results in a loss of deep pain sensation in bones and joints.
Using a genetically modified model NGF is replaced with human mutated NGF we are examining the role of NGF in joint health. While a role of NGF in pain signaling has been confirmed in patients with bone cancer and osteoarthritis, as well as models of the same conditions, the role of NGF in RA pain and bone erosion is not known.
The overall aim of this project is to advance our understanding of the link between NGF, bone homeostasis and pain using both evoked pain and non-evoked pain tests to asses deep pain sensation, as well as molecular and imaging techniques.
Mechanisms of chronic pain in osteoarthritis and low back pain
In collaboration with the Department of Clinical Neuroscience, we perform the clinical and molecular characterization of chronic pain in Swedish patient cohorts with OA and low back pain.
In this project, we are particularly interested in mechanisms behind neuroinflammation which might contribute to neuropathic pain and potential cross-talk between inflammatory mechanisms in CNS and on the periphery. We use molecular profiling of patient tissues to identify pro-inflammatory substances that can contribute to pain chronification and combine this approach with functional validation in experimental models to identify novel targets for pain therapies.

Group members
- Camilla Svensson, Professor, research group leader
- Alex Bersellini Farinotti, Postdoc
- Alexandra Jurczak, PhD student
- Emerson Krock, Postdoc
- Nilesh Agalave, doctoral student
- Kim Kultima, Affiliated to research
- Zerina Kurtovic, PhD student
- Harald Lund, Postdoc
- Joana Menezes, PhD student
- Lydia Moll, PhD student
- Carlos Morado Urbina, PhD student
- Resti Rudijito, PhD student
- Katalin Sandor, Senior researcher
- Nils Simon, PhD student
- Zhenggang Wang, visiting PhD student
- Francisca Castillo, Research assistant
Previous group members
- Simone Codeluppi, assistant professor
- Diana Nascimento, post doc
- Duygu Bas, post doc
- Sally Abdelmoaty, post doc
- Ada Delaney, post doc
- Katarzyna Rogoz, post doc
- Camilla Ultenius, post doc
- Jungo Kato, post doc
- Shibu Krishnan, post doc
- Tianle Gao, post doc
- Joshua Gregory, post doc
- Payam Emami, postdoc
- Vinko Palada, postdoc
- Freija ter Heegde, postdoc
- Gustaf Wigerblad, doctoral student
- Jie Su, doctoral student
- Teresa Fernandez-Zafra, doctoral student
- Anya Finn, doctoral student
- Ebba Gregory, doctoral student
- Kristina Ängeby-Möller, doctoral student
- Ahmad Shareef, research assistant
- Max Larsson, researcher
- Jaira Villareal Salcido, master student
- Merna Bitar, master student
- Nicole Nova, master student
- Johanna Pettersson, master student
- Fatima Rhordo, master student
- Sara Sadek, bachelor student
- Pia Sandberg, bachelor student
- Hanna Steurer, bachelor student
- Azar Baharpoor, Biomedical analyst
- Yuki Nomura, Affiliated to research
Selected publications
Characterization of neuroinflammation and periphery-to-CNS inflammatory cross-talk in patients with disc herniation and degenerative disc disease.
Palada V, Ahmed AS, Finn A, Berg S, Svensson CI, Kosek E
Brain Behav. Immun. 2019 Jan;75():60-71
Exploring the transcriptome of resident spinal microglia after collagen antibody-induced arthritis.
Fernandez-Zafra T, Gao T, Jurczak A, Sandor K, Hore Z, Agalave NM, et al
Pain 2019 Jan;160(1):224-236
Spinal injection of newly identified cerebellin-1 and cerebellin-2 peptides induce mechanical hypersensitivity in mice.
Sandor K, Krishnan S, Agalave NM, Krock E, Salcido JV, Fernandez-Zafra T, et al
Neuropeptides 2018 Jun;69():53-59
Autoantibodies Hurt: Transfer of Patient-Derived CASPR2 Antibodies Induces Neuropathic Pain in Mice.
Hunt MA, Nascimento DSM, Bersellini Farinotti A, Svensson CI
Neuron 2018 02;97(4):729-731
Phenotypic changes in dorsal root ganglion and spinal cord in the collagen antibody-induced arthritis mouse model.
Su J, Gao T, Shi T, Xiang Q, Xu X, Wiesenfeld-Hallin Z, et al
J. Comp. Neurol. 2015 Jul;523(10):1505-28
Spinal HMGB1 induces TLR4-mediated long-lasting hypersensitivity and glial activation and regulates pain-like behavior in experimental arthritis.
Agalave NM, Larsson M, Abdelmoaty S, Su J, Baharpoor A, Lundbäck P, et al
Pain 2014 Sep;155(9):1802-13
Collagen antibody-induced arthritis evokes persistent pain with spinal glial involvement and transient prostaglandin dependency.
Bas DB, Su J, Sandor K, Agalave NM, Lundberg J, Codeluppi S, et al
Arthritis Rheum. 2012 Dec;64(12):3886-96
K/BxN serum transfer arthritis as a model of inflammatory joint pain.
Christianson CA, Corr M, Yaksh TL, Svensson CI
Methods Mol. Biol. 2012 ;851():249-60
Full list of publications

Group activities
Research retreats

