Oral Biology and Medicine Group
Research projects of the Oral Biology and Medicine Group take a multi-disciplinary translational approach to address diseases affecting the soft tissues of the mouth. Through clinico-histopathological research, we are working to improve oral conditions and lesion diagnostics, with the aim to personalize management in orofacial medicine.
We focus on strengthening pathologist/clinician interactions by providing provide rapid accurate oral histological assessments for clinical decision-making to fulfil this goal.
Principally, we focus on the complications that result following hematopoietic stem cell transplantation in the form of Graft versus Host Disease (GVHD), which is a major cause for morbidity and mortality (~70%) in these patients. Oral manifestations are one of the most common and debilitating complications, with a diverse spectrum of clinical features, including mucosal lesions, salivary gland dysfunction and restricted mouth opening. Patients may experience mucosal sensitivity, oral pain, increased caries risk, malnutrition and problems speaking and long-term, complications include secondary malignancies and mortality. Current treatments alleviate symptoms but due to a general lack of understanding of oral chronic (c)GVHD, from clinical signs to histopathological diagnoses, the development of treatment regimens is slow. The research group is focusing their efforts on trying to improve clinical diagnoses by incorporating histopathological guidelines for oral cGVHD pathogenesis together with elucidating the underlying disease mechanisms.
In addition, studies investigating other pre-malignant and malignant oral diseases are ongoing, including mucositis, oral lichen planus, leucoplakia.
In response to the shortage of qualified oral pathologists and high inter-assessor variation, machine learning, and in particular deep learning methodologies are being developed to provide unique tools to increase digital diagnostic capabilities, precision and accuracy. Deep learning algorithms are applied for predictive oral lesion diagnostics to create prognostic and treatment application models.
Group members
- Rachael Sugars, BSC (Hons). PhD. Associate Professor
- Karin Garming Legert, DDS. PhD. Assistant Professor
- Sabrina Svedjevik DDS. Doctoral Student
- Victor Tollemar, DDS. PhD. Orofacial Medicine Resident
- Helena Arvidsson, DDS. Lic. Affiliated to research
- Nikolce Tudzarovski, MSc
Histopathological diagnostics of oral cGVHD
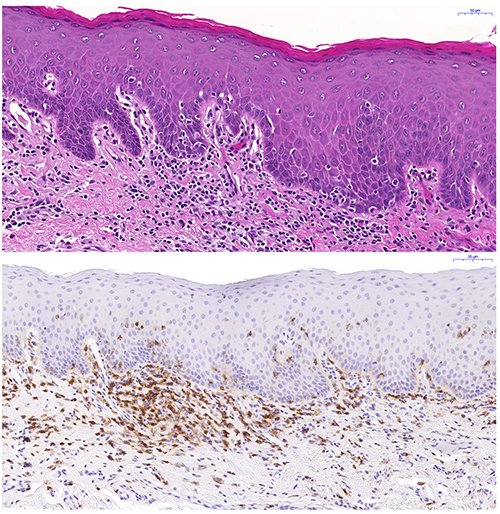
We aim to define the histopathological alterations of cGVHD in oral mucosal tissues and salivary glands. In a large cohort, we have devised a histological severity grading scheme for oral mucosal cGVHD lesion diagnostics, and we are also grading the pathological presentation of salivary gland cGVHD manifestations. In both, oral mucosa and salivary gland tissues we are assessing their immunological infiltrate and using digital quantitative immunohistochemistry to relate the histopathological presentation to clinical disease severity.
PI: Rachael Sugars
Group members: Victor Tollemar; Sabrina Svedjevik; Helena Arvidsson; Nikolce Tudzarovski; Karin Garming Legert
Collaborators: Professor Gunnar Warfvinge (Malmö Odontology Faculty); Dr. Johan Lundström (Orofacial Medicine, Public Dental Health Service, Huddinge); Professor Katarina Le Blanc (Laboratory Medicine KI)
Clinical staging of cGVHD with aligned histopathological presentation
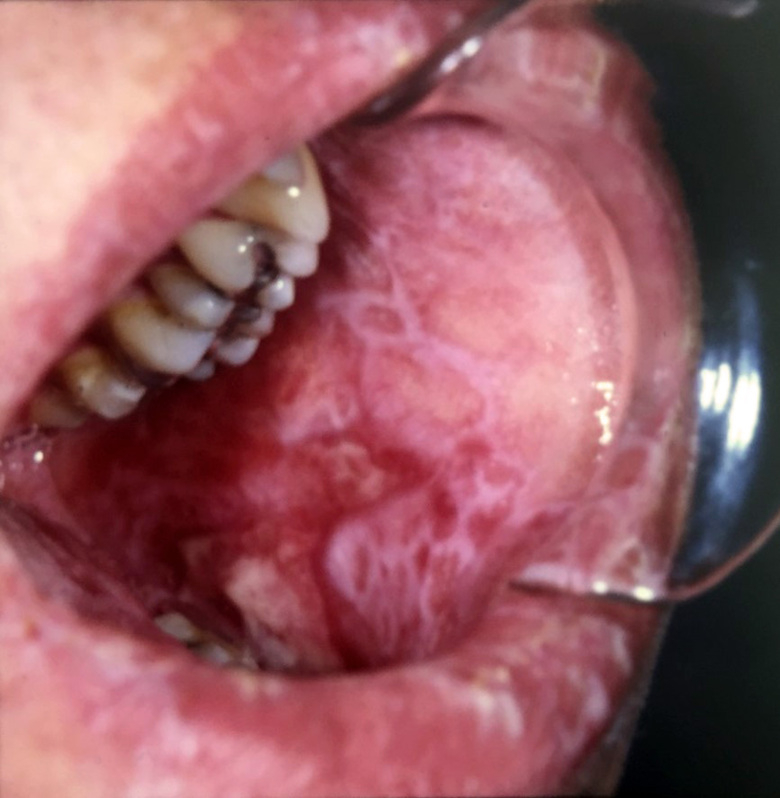
In this project, we aim to correlate clinical signs and symptoms of oral cGVHD with histopathological features and systemic issues, to define clearer oral mucosal and salivary cGVHD diagnostic criteria. We are assessing the current clinical diagnostic tools for oral cGVHD, with respect to other complications, such as infections and any development of secondary cancer. Altered clinico-histopathological screening will lead to improved management and treatment options. In conjunction, we are employing our new histological grading modules for oral mucosa and salivary gland oral cGVHD to validate their diagnostic potential and coupling them to the clinical presentation, with the goal to provide improved diagnostics.
PI’s: Rachael Sugars & Karin Garming Legert.
Group members: Victor Tollemar; Sabrina Svedjevik; Nikolce Tudzarovski
Collaborators: Dr Johan Karlsson Törlén (CAST, Karolinska University Hospital); Professor Naom Yaron ( School of Dentistry, Tel-Aviv University) and Professor Matteo Bottai (Biostatistics, KI).
Disease mechanisms and molecular biomarkers in oral cGVHD
Our knowledge of oral cGVHD is limited and so studies are exploring the underlying disease mechanisms, which has facilitated the search for diagnostic, prognostic or predictive biomarkers. These are crucially needed as an adjunct to clinical and histological criteria, to allow classification of patients into risk groups, refine diagnosis, predict those at risk of developing cGVHD or estimate patient responses to therapy.
PI: Rachael Sugars.
Group members: Helena Arvidsson; Victor Tollemar; Nikolce Tudzarovski; Karin Garming Legert.
Collaborators: Professor Gunnar Warfvinge (Malmö Odontology Faculty); Dr. Robert Heymann (Oral Diagnostics and Surgery KI); Dr. Johan Lundström (Medical Dentistry, Public Dental Health Service, Huddinge).
Artificial intelligence (AI) driven digital pathology for the assessment of oral pathology tissues
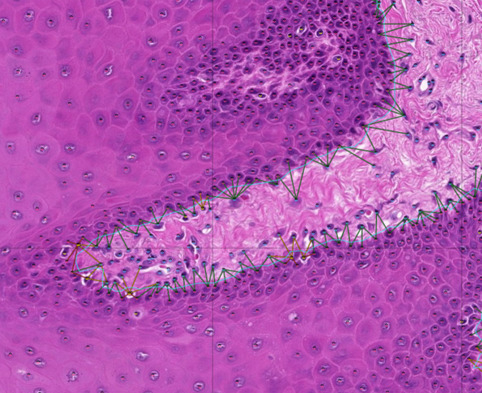
Recent advancements in AI, in particular machine learning can now be accurately trained to perform challenging prediction tasks for medical image classification. These have the potential to rapidly become a complement to diagnostic pathology. Using our extensive image dataset we are exploring modern deep learning-algorithms that “learn” predictive models directly from the raw data (supervised learning) for feature recognition and histological patterning. We have developed a pipeline to combine convolutional neural networks for cell identification and location, with graph neural networks to model the tissue topography, such as the location and integrity of the basement membrane.Through this combination we are able to accurately map histological and topological features of a tissue. The long-term goal of this project is to integrate all components of histological modules with disease pathophysiology, and prognostic/diagnostic biomarkers into an oral digital pathology platform.
PI: Rachael Sugars
Group members: Sabrina Svedjevik; Helena Arvidsson; Victor Tollemar; Nikolce Tudzarovski
Collaborators: Professor Karl Meinke (Division of Theoretical Computing Sciences, KTH), Professor Gunnar Warfvinge (Malmö Odontology Faculty); Dr. Johan Lundström (Orofacial Medicine, Public Dental Health Service, Huddinge)
Research support
- Cancerfonden (Swedish Cancer Society)
- Catherine Everts Research Foundation
- Region Stockholm/KI – SOF
- Region Stockholm/KI – ALF Medicine
- The Swedish Dental Association
- Clinical Scientist Program KI
Publications
Links to the publications can be obtained through the group members profile pages.
