Our research
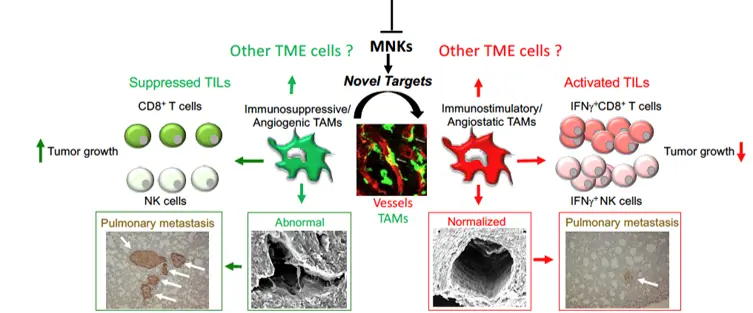
Our research primarily focuses on tumor immunology, with a particular emphasis on understanding the behavior of Tumor-Associated Macrophages (TAMs) and is dedicated to finding novel targets that reprogram immunosuppressive and pro-metastatic Tumor-Associated Macrophages into an anti-tumor phenotype. Our lab employs state-of-the-art molecular biology techniques, mouse models, and patient samples to uncover mechanisms that regulate TAM phenotypes. By targeting these mechanisms, the overarching aim is to reprogram pro-tumoral TAMs into an anti-tumoral state, affecting various components of the non-cancer cells residing in the tumor microenvironment, ultimately inhibiting tumor growth and metastasis (Figure 1).
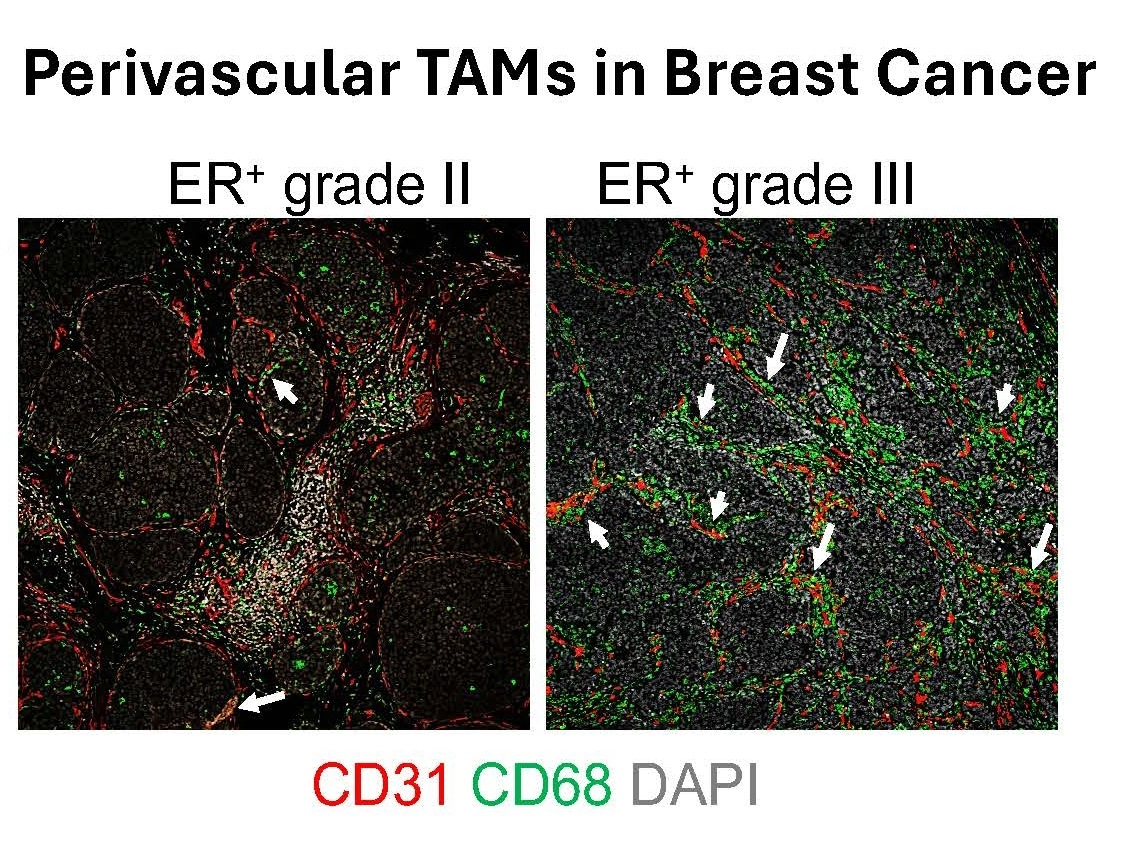
Accumulation of Tumor-Associated Macrophages (TAMs) is associated with tumor progression and poor survival in most cancers, including breast cancer. TAMs are plastic cells that exhibit a spectrum of states spanning from an anti-tumor/immunoactivating (sometimes referred to as M1-like) to a pro-tumor/immunosuppressive (sometimes referred to as M2-like) phenotype. We proposed already in 2011 that TAMs of an anti-tumoral phenotype located in the vicinity of tumor vessels (perivascular TAMs) promote vessel normalization that hampers metastatic dissemination. These TAMs further stimulate activation of cytotoxic T cells that kill tumor cells, resulting in suppression of tumor growth. Importantly, pro-tumoral perivascular TAMs in breast cancer (BC) are associated with poor prognosis and increased risk of distant metastasis (Figure 2).
In this respect, we recently illuminated a new mechanism whereby selective modification of mRNA translation regulates TAM immunosuppressive functions. Further mechanistic studies revealed that these functions were controlled by the eukaryotic initiation factor (eIF) 4E and the mitogen-activated protein kinase (MAPK) interacting protein kinase (MNK) signaling pathways, which regulate which mRNAs will be translated. Additionally, preliminary data indicate that MNKs are involved in controlling the pro-tumor functions of perivascularTAMs.
Our overall aim is to understand how TAM immunosuppressive and pro-metastatic functions are regulated and if these TAMs can be used as mechanistic, predictive and prognostic tools for different solid tumors, including breast cancer, adult and pediatric sarcoma, and other solid tumors.
Current projects
- Studying the cross-talk between TAMs phenotypes and the Tumor Microenvironment
- Metabolic pathways that regulate TAM phenotypes.
- Exploiting TAM subtypes for chemotherapy efficacy.
- TAMs as predictive and prognostic tools in breast cancer, adult and pediatric sarcoma and other solid tumors
Funding
- Swedish Research Council (VR)
- Swedish Cancer Society
- Cancer Society in Stockholm
- Karolinska Institutet Funds
- Tornspiran
- Wenner-Gren
- The Swedish Childhood Cancer Foundation
- KID
